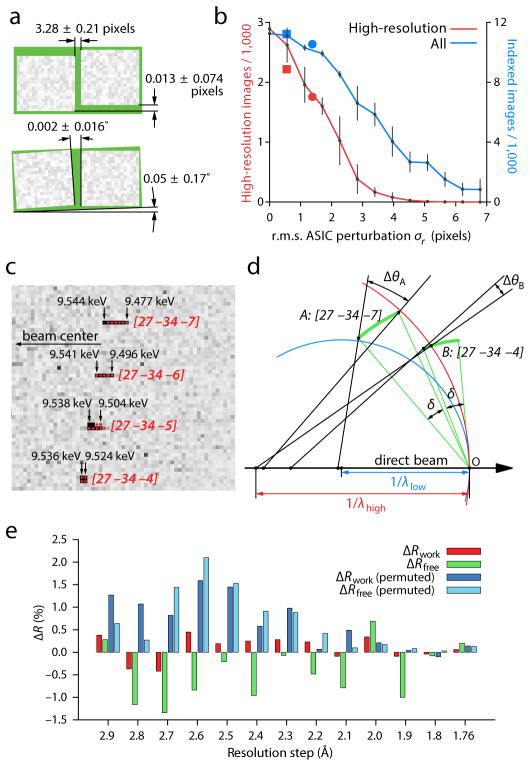
Figure 2. Calibration and validation.
(a) Aggregate relative positions (top) and rotations (bottom) of 32 pairs of application-specific integrated circuits (ASICs), each pair bump-bonded to a pixel array sensor of the CSPAD detector. The two ASICs on each sensor are manufactured to be aligned along the long axis, separated by a 3.0-pixel gap. These calibration results bear out this expectation within the tolerances shown. (b) Impact of positional accuracy on the indexing and integration success rate. Perturbing the ASICs away from their true positions reduces both the total number of indexed images (blue) and the number of images that contain successfully integrated reflections at high (1.8–2.2 Å) resolution (red). Error bars are the standard deviation from five different sets of perturbations drawn from a twodimensional normal distribution with a standard deviation σr. Separate perturbations were drawn for each ASIC. Squares: failure to apply final subpixel corrections from iterative least squares refinement. Circles: failure to apply nearest-whole pixel corrections. (c) Detail of four Bragg reflections on a thermolysin diffraction pattern, showing pronounced (seven pixel) radial elongation for the [27 –34 –7] reflection and lesser elongation for those nearby. Solution of Bragg’s law for each pixel (black arrows) identifies the spread of photon energies that contribute to each reflection. Red disks delineate integration masks from a three-parameter model with wavelength limits λhigh = 1.297 (9.556 keV) and λlow = 1.313 (9.443 keV), and full-width mosaic spread δ = 0.174°. (d) Reciprocal space diagram indicating how different-shaped reflections arise. Reciprocal lattice points (arcs) all have a constant angular extent δ due to their mosaic spread. Points are in reflecting condition if they are within the zone between the high-energy (red) and low-energy (blue) Ewald spheres. Therefore, a greater fraction of the [27 –34 –7] mosaic distribution is within the reflecting condition, leading to a reflection that subtends a greater radial angle Δθ. (e) Paired refinements of the thermolysin structure. Red and green bars indicate the change in Rwork and Rfree, respectively, as higher-resolution data are added to the refinement. Dark and light blue bars show changes to the R-factors when the newly added high-resolution structure factors are randomly permuted. The data are interpreted as containing statistically significant signal for the resolution shells where ΔRfree is continuously negative, i.e. out to 2.1 Å.