Abstract
The nutritional composition and antioxidant activity (in aqueose and solvent extracts) of two medicinal plants of Iranian origin Borage (Echium amoenum) and Valerian (Valerian officinalis) used as tea were determined. Samples were analyzed for antioxidant components viz. polyphenols, vitamin C, β carotene, flavonoids, anthocyanins and tannins. Antioxidant assays such as free radical scavenging activity, reducing power and total antioxidant activity were carried out for ethanol, methanol, acetone, 80% methanol and 80% ethanolic extracts. In borage highest and least activity was observed in water and acetone extract respectively in all assays. In Valerian, 80% methanolic extract showed highest activity in reducing power and free radical scavenging activity assay. Total polyphenols in borage and valerian were 1,220 and 500 mg in ethanolic extracts and 25 and 130 mg in acetonic extracts respectively. Total carotenoids and vitamin C contents were 31.6 and 133.69 mg and 51.2 and 44.87 mg for borage and valerian respectively. Highest amount of tannins were extracted in 80% methanolic extract. It can be concluded that borage and valerian exhibited antioxidant activity in all extracts. The antioxidant activity could be attributed to their polyphenol and tannin and flavonoids contents. In all assays borage showed higher activity than valerian.
Keywords: Anthocyanin, Echium amoenum, Valerian officinalis, Flavonoids, Plant, Polyphenols
Introduction
The past decade has observed a remarkable resurgence in the interest and use of medicinal plant products. According to World Health Organization ~80% of the earth inhabitants rely on traditional medicine for their primary health care needs and most of this therapy involves the use of plant extracts or their active components. Until synthetic drugs were developed in nineteenth century, herbs were the basis for nearly all medicinal therapy. Herbs are found in 40% of prescriptions and the interest for use of herbal remedies instead of chemical drugs is increasing because of lesser side effects (Craig 1999).
Heart disease continues to be a major cause of death; cancer, osteoporosis and arthritis remain highly prevalent. Though genetics play a major role in the progress of diseases mentioned, by and large most are considered preventable or could be minimized by activity, a proper diet, physical activity, weight management and a healthier lifestyle including environment. Functional foods can prevent or delay the onset of chronic diseases as well as provide basic nutritional requirements (Medoua et al. 2009). Nutraceuticals (or functional foods) which contain phytochemical constituents can have long-term health promoting or medicinal qualities. Although the distinction between medicinal plants and nutraceuticals can sometimes be indistinguishable, a primary characteristic of the latter is that nutraceuticals have a nutritional role in the diet and the benefits to health may arise from long-term use as foods (Korver 1998).
In contrast, many medicinal plants exhibit specific medicinal actions without serving a nutritional role in the human diet and may be used in response to specific health problems over short- or long-term intervals. For many of the medicinal plants of current interest, a primary focus of research to date has been in the areas of phytochemistry, pharmacognosy, and horticulture. In the area of phytochemistry, medicinal plants have been characterized for their possible bioactive compounds, which have been separated and subjected to detailed structural analysis. Research in the pharmacognosy of medicinal plants has also involved assays of bio-activity, identification of potential modes of action, and target sites for active phytomedicinal compounds.
There is scientific evidence that dietary antioxidants play an important role in human health (Liu 2003). Free radicals have been associated in many diseases such as diabetes, aging, neurodegenerative diseases, atherosclerosis, carcinogenesis, inflammation and metabolic disorders, (Ames et al. 1993; Halliwell and Gutteridge 1999; Yu 1995) and antioxidants can react with free radicals to neutralize its oxidative properties and prevent cell wall damage and other detrimental effects. The major components that contribute to the antioxidant capacity of foods are polyphenols, carotenoids and vitamin C and E (Medoua et al. 2009) and in several studies antioxidant activity of foods and isolated components has been reported (Wojdylo et al. 2007; Lako et al. 2007; Podsedek 2007).
Antioxidants are widely employed to increase the shelf life of lipid and lipid containing food products and to reduce wastage by inhibiting and delaying oxidation and they are also used as a food preservative (Cherbuliez and Domerego 2003). There are two kinds of antioxidants, natural and synthetic antioxidants. Restriction on the use of synthetic antioxidants is being imposed due to their carcinogenicity (Grice 1988). Vitamin C, carotenoids and tocopherols are most important natural antioxidants which are also used in industry to improve shelf life of product. Other than these antioxidants, fibre, polyphenols, flavonoids, conjugated isomers of linoleic acid, soy protein, isoflavones, selenium, chlorophyllin, glutathione, protease inhibitors, sulphides, and catechin are natural antioxidants found. Antioxidants or ingredients having antioxdative properties are used widely to improve food stability. With the focus to find alternatives for synthetic food ingredients, natural substances having antioxidative properties need to be further explored (Sachindra et al. 2010)
Since each of the components are different in nature, different solvent system have been used for extraction of sample for estimation of antioxidant compound and antioxidant activity of sample (Chavan et al. 2001). Borage (Borago officinalis) and valerian (Valerian officinalis) are two popular herbs which are used regularly in Iran as a stress relief decoction separately or in combination. In folk medicine of Iran the flowers of borage have been used as demulcent, anti-inflammatory and analgesic, anxiolytic, and sedative (Wretensjo et al. 1990). Two separate experimental studies in mice have shown its anxiolytic effect (Shafaghi et al. 2002; Kast 2001). The main modern and historical uses of valerian are as sedative and anxiolytic. Other uses of valerian include the treatment of headaches, palpitation, high blood pressure, irritable or spastic bowel, menstrual cramps, childhood behaviour problems and learning disability (Klich 1975; Friedlander et al. 2003; Caron and Riedlinger 2002).
In view of the importance of antioxidant potential of herbs, we were interested in evaluating the antioxidant activity and nutritional properties of valerian and borage, as little is known about their antioxidant components and activity. Water, aqueous mixtures of ethanol, methanol and acetone are commonly used to extract plants (Sun and Ho 2005) since it is not known that which solvent system is more useful for extracting antioxidant components and estimating antioxidant activity, we selected water, methanol, ethanol, acetone and also 80% methanol and 80% ethanol as extraction media.
Material and methods
Plant material
Fresh petals of borage (Echium amoenum) belonging to Borago officinalis family and valerian root (Valerian officinalis) belonging to valerianaceae were collected from a farm near Ardebil (a city in north western of Iran), washed with distilled water and dried in oven at 40 °C, for 36 h ground and stored in air tight container under refrigeration at 4 °C.
Chemical reagents and solvents
The chemicals used for the study were procured from Qualigen Company Mumbai, India, Himedia Company, Mumbai, India and Sigma Company, USA. They were all of analytical grade. Glass double distilled water, methanol, ethanol, acetone, 80% methanol and 80% ethanol were used for extraction.
Sample preparation for determination of antioxidant activity
250 mg of samples were mixed with 25 ml of extraction media and extracted for 3 h, centrifuged at 4,000 rpm for 20 min, and passed through filter paper (Whatman No.1) to get clear extract. Water extracts were taken at 30 °C and 100 °C and solvent extracts at 10 to 12 °C.
Proximate composition and trace element estimation
Nutritional composition –Moisture, total protein, fat, ash, vitamin C (AOAC 2005), dietary fibre (Asp et al. 1983), calcium (Oser 1965), phosphorus, iron (Wong 1928) of all the samples were estimated with standard techniques.
Moisture (9261.12,41.1.02)
A known amount of sample was taken in a petri plate and dried in an oven. The dry weight of the sample was determined by repeated consistent weighing.
Total protein (960.52,12.1.07)
The estimation of nitrogen was done by Kjeldhal method that depends on the fact that organic nitrogen when digested with sulphuric acid in the presence of a catalyst is converted into ammonium sulphate. Ammonia liberated by making the solution alkaline is distilled into a known volume of a standard acid, which is then back titrated. The protein content was obtained by multiplying the nitrogen value with 6.25.
Ash (942.05,4.1.10)
The greyish white residue that remains after the food sample taken in a silica crucible is charred on a hot plate, incinerated in a muffle furnace at 600°C for 3–5 h and weighed is total ash.
Total fat (948.22,40.1.05)
Fat is estimated as crude ether extract of the dry material. The dry sample (5–10 g) is weighed accurately into a thimble and plugged with cotton. The thimble is placed in a Soxhlet apparatus and extracted with petroleum ether for about 16 h. The ether extract is filtered into a weighed conical flask. The flask containing the ether extract is washed 4–5 times with small quantities of ether and the washings are also transferred. The ether is then removed by evaporation, the flask with the residue dried in an oven at 80 - 100°C, cooled in a dessicator and weighed.
Total iron
To a known volume of mineral solution (taken from the total ash as described previously), 1.0 mL each of 30% H2SO4 and 7% potassium persulphate solution and 1.5 mL of 40% potassium thiocyanate solution were added with thorough mixing. The red colour that developed was measured within 20 min at 540 nm. Similarly a standard curve was generated by using ferrous ammonium sulphate. The iron content of sample was then read off from the standard curve.
Phosphorous
Phosphorus was determined by treating the ash solution with ammonium molybdate and the phosphomolybdate thus formed was reduced and the blue colour that developed was estimated colorimetrically at 660 nm Calcium was determined by precipitating it as calcium oxalate and titrating the solution of oxalate in dilute sulphuric acid against standardized KMnO4.
Calcium
Calcium was precipitated as calcium oxalate. The precipitate is dissolved in hot dilute H2SO4 and titrated against standard potassium permanganate. Pipette an aliquot (25 ml) of the ash solution obtained by dry ashing to a 250 ml conical flask. Dilute to 150 ml with water. Add a few drops of methyl red indicator and neutralize the mixture with ammonia till the pale pink color changes to yellow. Heat the solution to boiling point, add 10 ml of ammonium oxalate and boil again for a few min. Add glacial acetic acid till color of the mixture is distinctly pink. Allow to stand at room temperature for at least 4 h or preferably overnight. Filter through Whatman No. 42 paper and wash with warm water, till the filtrate is oxalate free. (Since HCl had been used for preparing the ash solution, it is convenient to test for the absence of chloride using AgNO3). Add 5–10 ml of dilute H2SO4 on the filter paper, break the point of the filter paper with a pointed glass rod and transfer it to the conical flask. The solution is heated to 70°C and titrated against 0.01N KMnO4 to a permanent pale pink color.
Total dietary fiber
Total dietary fibre (Asp et al. 1983) was determined by enzymatic-gravimetric method wherein the digestible starch and protein was removed using enzymes [heat stable amylase (termamyl), pepsin, pancreatin] and the sample was filtered through a G2 crucible using celite as a filtering aid, oven dried (100 °C) and weighed till two consecutive weights were constant. Corrections for protein and minerals in the residue were accordingly made.
Ascorbic acid
It was estimated by a visual titrimetric method using 2, 6-dichlorophenol indopenol dye which is blue in alkaline solution and red in acid solution and turns colourless when reduced by ascorbic acid.
The ash solutions were prepared with wet digestion (Ranganna 1986) and analyzed for zinc, copper, chromium and manganese using atomic absorption spectrophotometer (AAS) (GBC Scientific equipment, Australia). Instrument parameters such as resonant wavelength, slit width and air-acetylene flow rate that are appropriate for each element were selected (AOAC 2000).
Antioxidant components estimation
Total phenolic compound analysis
Total polyphenol content was estimated using Folin-Ciocalteu (FC) assay which is widely used in routine analysis (Wright et al. 2000; Atoui et al. 2005). A known amount of extract (10 mg/ml) was mixed with 1.0 ml of FC reagent and 0.8 ml of Na2Co3 was added and the volume was made up to 10 ml using water- methanol (4:6) as diluting fluid. Absorbance was read at 740 nm after 30 min using spectrophotometer (Systronics, UV/VIS-108, Systronics, 89–92, Industrial Area, Naroda, Ahmedabad – 382330). Tannic acid (0–800 mg/L) was used to produce standard calibration curve. The total phenolic content was expressed in mg of tannic acid equivalents (TAE)/100 g of sample (Matthaus 2002).
Estimation of total flavonoids
The total flavonoid content was estimated using the Dowd method as adapted by (Arvouet-Grand et al. 1994). A 5.0 ml of 2% aluminium trichloride (AlCl3) in methanol was mixed with the same volume of the extract solution (10 mg/ml). Absorption readings at 415 nm using Perkin Elmer UV–VIS spectrophotometer were taken after 10 min against a blank sample consisting of extract solution with 5.0 ml methanol without AlCl3. The total flavonoid content was determined using a standard curve with quercetin. Total flavonoid content is expressed as g of quercetin equivalents/100 g of sample.
Anthocyanin estimation
The procedure involved extraction of the anthocyanins with ethanolic HCL and measurement at wavelength of maximum absorption. 2.0 g of sample was blended with 70 ml of ethanolic HCL. Extract was stored overnight in a refrigerator at 4 °C. It was filtered on a Whatman No.1 paper and made up to 100 ml. To prepare extract for spectrophotometric measurement, 1.0 ml of sample was diluted to 10 ml. OD was taken after storing the sample in dark for 2 h and colour was measured at the maximum absorption (545 nm) (Ranganna 1986).
Total tannin estimation
Colorimetric estimation of tannins is based on the measurement of blue colour formed by the reduction of phosphotungstomolybdic acid by tannin like compounds in alkaline solution (Ranganna 1986). A known amount of extract was mixed with 5.0 ml of Folin- Denis reagent (FD) and Na2Co3 solution and made up to 100 ml, mixed well and absorbance was read at 760 nm after 30 min using spectrophotometer. Total tannin content was expressed as mg tannic acid equivalent/100 g of sample.
Determination of antioxidant Activity of different extract
Radical scavenging activity by DPPH (2, 2-diphenyl-1-picrylhydrazyl)
Effect of different extracts on DPPH free radical was measured according to Lee et al. (1996). Positive control (standard) was prepared by mixing 4.0 ml of ascorbic acid (0.05 mg/ml) and 1.0 ml of DPPH (0.4 mg/ml) for water extract, and negative control (blank) was prepared by mixing extract base (water/methanol/ethanol/acetone) with 1.0 ml of DPPH.
Four different concentration of extract was mixed with 4.0 ml of DPPH, the volume made up to known volume and mixed well and left to stand at room temperature in a dark place for 30 min. Absorbance was read using a spectrophotometer at 520 nm. The ability of extract to scavenge DPPH was calculated using the following equation.
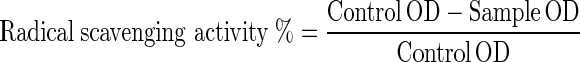 |
Reducing power
A spectrophotometric method was used for the measurement of reducing power. Different concentration of extracts was mixed with 2.5 ml phosphate buffer (0.2 M, pH 6.6) and 2.5 ml of 1% potassium ferricyanide (10 mg ml-1). The mixture was incubated at 50 °C for 20 min, then rapidly cooled, mixed with 2.5 ml of 10% trichloroactic acid and centrifuged at 6,500 rpm for 10 min. The supernatant (2.5 ml) was mixed with distilled water (2.5 ml) and then ferric chloride (0.5 ml, 0.1%) was added and allowed to stand for 10 min. The absorbance was read spectrophotometrically at 700 nm (Oyaizu 1986).
Total antioxidant activity by phosphomolybdenum method
0.1 ml of extract (10 mg/ml) was mixed with reagent solution (0.6 M sulphuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate in 100 ml), tubes capped and incubated in boiling water bath at 95 °C for 90 min. Then cooled to room temperature and absorbance was read at 695 nm with spectrophotometer against blank. Water soluble antioxidant capacity expressed as equivalent of ascorbic acid (μmol/g of sample) (Prieto et al. 1999).
Statistical analysis
Data were expressed as mean ± SD. in all experiments. To determine the extent of association between antioxidant activity and antioxidant components in different extracts, data were subjected to correlation coefficient in Excel 2007.
Results and discussion
Nutritional composition
The nutritional composition of borage and valerian were determined with standard techniques and results are presented in Table 1. Fresh flower of borage was found to contain 88.9% moisture wheras the dry flower contained 9.9%. Protein content was found to be 7.3 and 4.3 and fat content was 1.2 and 1.1 g/100 g in borage and valerian respectively. The ash content of samples was high which shows the high mineral content of samples. As shown in Table 1 calcium, phosphorous and iron were all high at 766 mg, 303 mg and 252 mg/100 g for valerian and 704 mg, 356 mg and 68 mg/100 g for borage respectively. Total fibre of sample was estimated both in the form of soluble and insoluble fibre. Insoluble fibre was high in valerian (71.2%) and soluble fibre was high in borage (45%). Borage and valerian had 2.4 and 4.4 of zinc, 1.3 and 2.5 of copper, 1.6 and 10.6 of manganese and 30 and 230 of chromium as mg/100 g of sample respectively. Valerian was a better source of these trace minerals than borage. However, it is to be noted that these herbs are used as decoctions, hence only soluble nutrients could be made available through such extracts, and insoluble nutrients are left as residue. Since the mineral content is very high, they may provide fair amount of minerals.
Table 1.
Chemical composition of Borage and Valerian (per 100 g) (n = 4)
| Constituents | Borage | Valerian | constituents | Borage | Valerian |
|---|---|---|---|---|---|
| Moisture (g) | 9.9 ± 0.10 | 7.6 ± 0.11 | Ash (g) | 8.3 ± 0.40 | 8.3 ± 0.30 |
| Protein (g) | 7.3 ± 0.10 | 4.3 ± 0.10 | Calcium (mg) | 704 ± 0.30 | 766 ± 0.80 |
| Fat (g) | 1.2 ± 0.10 | 1.1 ± 0.08 | Phosphorous (mg) | 356 ± 0.93 | 303 ± 1.0 |
| Insoluble fibre (g) | 38 ± 0.50 | 71 ± 0.20 | Iron (mg) | 68 ± 0.70 | 252 ± 0.89 |
| Soluble fibre (g) | 45 ± 0.00 | 6.8 ± 0.10 | Zinc (mg) | 2.4 ± 0.00 | 4.4 ± 0.00 |
| Carbohydrate (g) By difference | 0 | 9 ± 0.05 | Copper (mg) | 1.3 ± 0.00 | 2.5 ± 0.00 |
| Vitamin C (mg) | 51.2 ± 0.50 | 44.9 ± 0.40 | Manganese (mg) | 1.6 ± 0.00 | 10.6 ± 0.00 |
| Total carotenoids (mg) | 31.6 ± 0.80 | 132.7 ± 0.10 | Chromium (mg) | 30 ± 0.00 | 230 ± 0.00 |
| Anthocyanin (mg) | 104.4 ± 0.25 | ND | - | - | - |
ND Not detected
Antioxidant components of both herbs were estimated in all 5 extracts and are presented in Table 2.
Table 2.
Antioxidant components (g/100 g) and total antioxidant activity (μmol/g) of Borage in different solvent extracts
| Constituents | Water (100 °C) | Water (30 °C) | Methanol | Ethanol | 80% Methanol | 80% Ethanol | Acetone |
|---|---|---|---|---|---|---|---|
| Borage | |||||||
| Yield of extract | 42.5 | 37.6 | 35.25 | 27.53 | 45.63 | 51.26 | 3.03 |
| Total Polyphenols | 1.54 ± 0.033 | 1.17 ± 0.028 | 0.450 ± 0.004 | 0.340 ± 0.005 | 1.120 ± 0.018 | 1.22 ± 0.019 | 0.025 ± 0.00 |
| Tannins | 2.47 ± 0.064 | 1.80 ± 0.03 | 1.16 ± 0.12 | 0.95 ± 0.038 | 2.45 ± 0.096 | 2.39 ± 0.137 | 0.17 ± 0.001 |
| Flavonoids | 4.54 ± 0.042 | 3.92 ± 0.092 | 0.82 ± 0.012 | 0.33 ± 0.001 | 1.10 ± 0.009 | 0.85 ± 0.009 | 0.30 ± 0.005 |
| Total antioxidant activity | 238,235 ± 111 | 229,706 ± 105 | 146,325 ± 75 | 89,705 ± 67 | 108,639 ± 38 | 70,000 ± 62 | 10,698 ± 30 |
| Correlation Coefficient [R2 Values] | Water Extract | Solvent Extract | - | ||||
| DPPH | Reducing Power | Total Antioxidant | DPPH | Reducing Power | Total Antioxidant | - | |
| Flavonoids | 1 | 1 | 1 | 0.74 | 0.95 | 0.243 | - |
| Polyphenols | 1 | 1 | 1 | 0.94 | 0.93 | −0.39 | - |
| Total tannin | 1 | 1 | 1 | 0.96 | 0.92 | −0.4 | - |
(Values are mean ± standard deviation of four replicates for each estimation. Polyphenols and tannins were expressed as tannic acid equivalent, flavonoids, as quercetin equivalent)
Total phenolic content
As shown in Table 2 borage exhibited higher phenolic content compared to valerian. In borage phenolic content was higher in hot water extract (1,540 mg/100 g) followed by room temperature water extract (1.17 g tannic acid equivalent/100 g). Acetonic extract showed least polyphenol content (0.025 g/100 g). Methanolic and ethanolic extract also showed lesser polyphenol content compared to 80% methanolic and 80% ethanolic extract. In 80% methanolic and 80% ethanolic extract values were closer to water extract. It can be due to high solubility of borage polyphenols in water. In valerian 80% ethanolic extract showed highest polyphenols (0.500 g/100 g) followed by 80% methanolic extract (0.380 g/100 g) and acetone extract showed least activity (0.130 g/100 g). Antioxidant activity of plant extract is usually linked to their phenolic content. Hydrogen donating characteristics of the phenolic compounds is responsible for the inhibition of free radical induced lipid peroxidation (Yen et al. 1993). Phenolic compounds are also known as high level antioxidants because of their ability to scavenge free radicals and give oxygen species such as singlet oxygen, superoxide free radicals and hydroxyl radicals (Hall 1997), though, it is well accepted that non phenolic antioxidants might also contribute to the antioxidant activity of plant extract (Hassimotto et al. 2005; Harish and Shivanandappa 2006). In a study researchers estimated total polyphenol content of 35 different herbs and medicinal plants in 80% methanolic extract. The polyphenol content was between 0.8 and 42.1 mg of gallic acid equivalent/g dry weight (dw) (Kahkonen et al. 1999). In our study when we calculate total polyphenols content in 80% methanolic extract/g of dw, borage showed 12.4 mg of TAE/g dw and valerian shows 4.112 mg of TAE/g dw.
Turkmen et al. (2006) reported polyphenol content (mg/g) of mate and black tea in different solvent extracts, the values being 30.5 and 64.2 in water extract, 1.8 and 2.6 in acetonic extract, 53.7 and 83.5 in 80% ethanolic extract, 2.1 and 4.8 in ethanolic extract, 56.0 and 85 in 80%methanolic extract and 13.5 and 35.5 in absolute methanolic extract in black tea and mate respectively. As data showed polyphenol content of black tea and mate varied in different extraction solvent and the order of polyphenol content was 80% methanolic extract >80% ethanolic extract > water > methanol > ethanol > acetone for both black tea and mate. But in our study order of polyphenol content of borage and valerian in different solvent was as follows respectively, water 100 °C > water 30 °C >80% ethanol >80% methanol > methanol > ethanol > acetone and 80% ethanol >80%methanol > water 100 °C > methanol > ethanol > water 30 °C > acetone. It can be due to different polyphenol components in our samples in comparison with what is found in black tea and mate.
Flavonoid content
Flavonoids were estimated in both samples in all extracts and data is presented in Table 2. In both borage and valerian flavonoids were higher in hot water extract followed by room temperature, 80% methanolic extract and 80% ethanolic extract. It ranged from 4.54 to 0.30 in borage and 3.03 to 0.3 g/100 g in valerian. Flavonoids were least in acetone extract in both valerian and borage.
Tannin estimation
Tannin was also estimated in all seven different extracts (Tables 2 and 3). Between two tested samples analysed tannin content of borage was more than valerian in all different extracts. As shown in Tables 2 and 3, it ranged from 0.16 mg to 2.47 mg for borage and 0.13 mg to 0.57 mg in valerian. Among solvents tested, hot water extract showed more tannin content in both samples and least was seen in acetone extract in both samples. In borage after hot water extract, 80% methanol and 80% ethanolic extract showed highest content, whereas in valerian, it was vice versa.
Table 3.
Antioxidant components (g/100 g) and total antioxidant activity (μmol/g) of Valerian in different solvent extract
| Constituents | Water (100 °C) | Water (30 °C) | Methanol | Ethanol | 80% Methanol | 80% Ethanol | Acetone |
|---|---|---|---|---|---|---|---|
| Yield of extract | 5.4 | 3.25 | 5.7 | 5.06 | 12.2 | 5.73 | 0.066 |
| Total Polyphenols | 0.315 ± 0.0034 | 0.185 ± 0.002 | 0.265 ± 0.003 | 0.250 ± 0.004 | 0.380 ± 0.006 | 0.500 ± 0.002 | 0.130 ± 0.003 |
| Tannins | 0.57 ± 0.087 | 0.43 ± 0.190 | 0.340 ± 0.076 | 0.3 ± 0.025 | 0.442 ± 0.044 | 0.454 ± 0.005 | 0.130 ± 0.014 |
| Flavonoids | 2.33 ± 0.101 | 3.03 ± 0.123 | 0.60 ± 0 | 0.48 ± 0 | 0.76 ± 0.018 | 1.05 ± 0.021 | 0.30 ± 0.004 |
| Total antioxidant | 31,985 ± 82 | 22,800 ± 102 | 64,117 ± 139 | 61,029 ± 99 | 98,970 ± 87 | 32,941 ± 55 | 32,463 ± 64 |
| Correlation Coefficient [R2 Values] | Water Extract | Solvent Extract | - | ||||
| DPPH | Reducing Power | Total Antioxidant | DPPH | Reducing Power | Total Antioxidant | - | |
| Flavonoids | −1 | −1 | −1 | 0.54 | 0.40 | 0.32 | - |
| Polyphenols | 1 | 1 | 1 | 0.45 | 0.52 | 0.38 | - |
| Total tannin | 1 | 1 | 1 | 0.50 | 0.65 | 0.67 | - |
(Values are mean ± standard deviation of four replicates for each estimation. Polyphenols and tannins were expressed as tannic acid equivalent, flavonoids, as quercetin equivalent)
Anthocyanin content
Anthocyanin in valerian could not be detected but borage showed high (104.4 mg/100 g) anthocyanin. Anthocyanin content of fruits has been reported and they contain varying amounts, for example Liu et al. (2002) reported anthocyanin content of raspberry to be between 0.17 and 57.0 mg/100 g, whereas for grapes it was reported to be 30–750 mg/100 g of by Bridle and Timberlake (1997).
Total antioxidant activity
Antioxidant activity was more in borage than valerian. In valerian 80% methanolic extract showed highest total antioxidant activity followed by methanolic extract. Water extract at room temperature showed least activity. Borage showed highest activity in hot water extract followed by room temperature water extract. Least activity was seen in acetonic extract.
For comparison of antioxidant component and antioxidant activity of borage and valerian T test was done and as it is shown in Table 4.
Table 4.
P value showing the significant differences between constituents of borage and valerian on application of student ‘T’ test
| Constituents | Water (100 °C) | Water (30 °C) | Methanol | Ethanol | 80% Methanol | 80% Ethanol | Acetone |
|---|---|---|---|---|---|---|---|
| Total Polyphenols | 0.000*** | 0.000*** | 0.000*** | 0.000*** | 0.000*** | 0.000*** | 0.000*** |
| Tannins | 0.018** | 0.025** | 0.055 ns | 0.018** | 0.015** | 0.015** | 0.078 ns |
| Flavonoids | 0.010** | 0.020 | 0.012 | 0.002** | 0.003** | 0.038** | 0.007** |
| Total antioxidant | 0.000*** | 0.000*** | 0.000*** | 0.000*** | 0.000*** | 0.000*** | 0.000*** |
*: P ≤ 0.05, **: P ≤ 0.01, ***: P ≤ 0.001
Antioxidant activity by reducing power assay
It has been reported that the reducing power of bioactive compounds is associated with antioxidant activity (Yen et al. 1993; Siddhuraju et al. 2002). Hence it is essential to determine the reducing power of phenolic constituents to explain the relationship between their antioxidant effect and their reducing power. The reducing power of different solvent extracts of borage and valerian is shown in Fig. 1. As illustrated in Fig. 1 part A, borage showed highest reducing power properties in hot water and room temperature water extract followed by 80% methanolic and 80% ethanolic extract. The least reducing power properties were exhibited by acetone extract whereas valerian showed highest reducing power properties in 80% methanolic and 80% ethanolic extract. Acetone extract showed least activity (Fig. 1 part B). As results support, extracts obtained by high polarity solvents are significantly more effective in reducing power and radical scavengers than those obtained by less polarity solvents. This specifies that antioxidant or active compounds of different polarity might be present in borage and valerian. Change in solvent polarity varies its ability to dissolve a selected group of antioxidant compound and persuade the antioxidant activity determination (Zhou and Yu 2004).
Fig. 1.
Reducing power of Borage (a) and Valerian (b) in different extracting media (Mean ± SD of six replicates). a Concentration of borage: A. 2,000 ppm, B. 4,000 ppm, C. 6,000 ppm and D. 8,000 ppm. b Concentration of valerian: A.2000 ppm, B. 4,000 ppm, C. 6,000 ppm, D. 8,000
Free radical scavenging activity
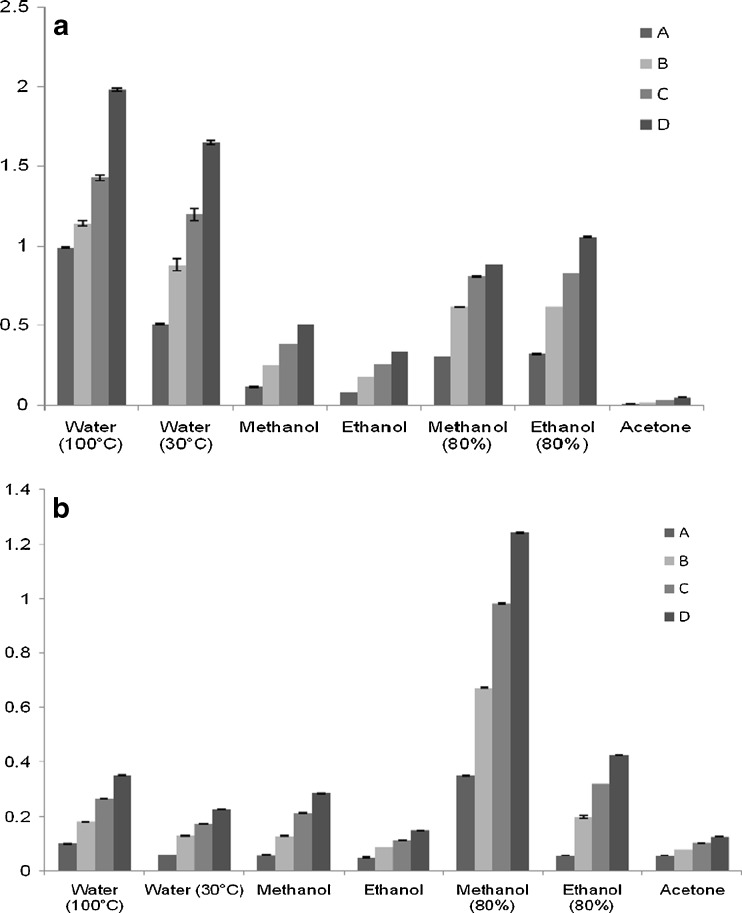
DPPH is a stable free radical in methanol or aqueous solution and accepts an electron or hydrogen radical to turn into stable diamagnetic molecule. It is usually used as a substrate to evaluate the antioxidative activity of antioxidants (Duh et al. 1999) thus we have estimated the antioxidant activity through free radical scavenging of borage and valerian. Free radical scavenging potency of samples is presented in Fig. 2.
Fig. 2.
Radical scavenging activity of Borage (a) and Valerian (b) in different extracting media (Mean ± SD of six replicates). a Concentration- Acetone extract: A: 5.0, B: 10.0, C: 15.0 and D: 20.0 mg of sample, all other extracts A: 0.4, B: 0.6, C: 0.8 and D: 1.0 mg of sample. b Concentration- 80% methanol: A: 0.4, B: 0.6, C: 0.8 and D: 1.0 mg of sample, all other extracts A: 2.0 mg, B: 4.0 mg, C: 6.0 mg, D: 8.0 mg of sample
As illustrated, valerian exhibited the highest radical scavenging activity in 80% methanolic extract followed by hot water extract and least activity in acetone extract. Borage showed highest activity in hot water extract followed by room temperature water extract and least activity was seen in acetonic extract.
Antioxidant components and activity are highly dependent on extracting solvent and concentration of solvent (Turkmen et al. 2006) but also vary within the samples. Many researchers have reported about the relationship between phenolic content and antioxidant activity. In some studies they found a correlation between the phenolic content and antioxidant activity (Velioglu et al. 1998) whereas others found no relationship (Kahkonen et al. 1999). In our study borage showed high correlation between antioxidant components, and antioxidant activity measured by reducing power and free radical scavenging method in both water (R2 = 1) and solvent extract but there were no correlation between total antioxidant activity and antioxidant components in solvent extract. In valerian both polyphenols and tannin showed high correlation with free radical scavenging activity, reducing power and total antioxidant activity in water extract (R2 = 1). Correlation was seen in solvent extract between tannin content and total antioxidant activity and reducing power R2 = 0.672 and R2 = 0.654 respectively. For borage acetonic extract was not included for correlation test as it had shown least activity in different concentrations. But there was no correlation between flavonoids content and antioxidant activity in valerian. Over 6,000 flavonoids have been identified in plant (Harborne and Williams 2000; Godevac et al. 2004) and antioxidant activity of many of the flavonoids against several oxidants have been studied by many authors (Ghasemzadeh et al. 2010; Fardet 2010; Wojdylo et al. 2007). It has been demonstrated that the antioxidant efficacy depends on structural features such as the number and position of the hydroxyl moieties on the ring systems and the extent by which unpaired electron in the oxidized phenolic intermediate can delocalize throughout the molecules (Lugasi and Hóvári 2003). Hence there is a possibility that all plant foods containing flavonoids may not exhibit antioxidant activity.
Antioxidant activity in borage extracts was substantially higher compared to valerian and significant differences were found between them. This can be correlated to antioxidant components which are significantly higher in borage in all extracts than valerian.
Conclusion
It can be concluded that borage and valerian both are good source of antioxidants apart from their medicinal properties, hence can be used as antioxidant supplements. In comparison with each other, borage contained higher antioxidant component and showed more activity than valerian. Both samples had higher content of water soluble antioxidant components as they exhibited higher activity in aqueous extract.
References
- Ames BN, Shigenaga MK,Hagen TM (1993) Oxidants, antioxidants, and the degenerative diseases of aging. In: Proceedings of the National Academy of Sciences, vol 90. vol 17. National Acad Sciences, p 7915 [DOI] [PMC free article] [PubMed]
- AOAC (2000) Official methods of analysis. 17th edn. Association of Official Analytical Chemists, Arlington, USA
- AOAC (2005) Determination of moisture, ash, protein and fat. 18th edn. Association of Official Analytical Chemists, Washington, USA
- Arvouet-Grand A, Vennat B, Pourrat A, Legret P. Standardisation d'un extrait de propolis et identification des principaux constituants = Standardization of a propolis extract and identification of the main constituents. J de Pharmacie de Belgique. 1994;49(6):462–468. [PubMed] [Google Scholar]
- Asp NG, Johansson CG, Hallmer H, Siljestrom M. Rapid enzymatic assay of insoluble and soluble dietary fiber. J Agric Food Chem. 1983;31(3):476–482. doi: 10.1021/jf00117a003. [DOI] [PubMed] [Google Scholar]
- Atoui AK, Mansouri A, Boskou G, Kefalas P. Tea and herbal infusions: their antioxidant activity and phenolic profile. Food Chem. 2005;89(1):27–36. doi: 10.1016/j.foodchem.2004.01.075. [DOI] [Google Scholar]
- Bridle P, Timberlake CF. Anthocyanins as natural food colours–selected aspects. Food Chem. 1997;58(1–2):103–109. doi: 10.1016/S0308-8146(96)00222-1. [DOI] [Google Scholar]
- Caron MF, Riedlinger JE. Valerian: a practical review for clinicians. Nutr Clin Care. 2002;2(4):250–257. [Google Scholar]
- Chavan UD, Shahidi F, Naczk M. Extraction of condensed tannins from beach pea (Lathyrus maritimus L.) as affected by different solvents. Food Chem. 2001;75(4):509–512. doi: 10.1016/S0308-8146(01)00234-5. [DOI] [Google Scholar]
- Cherbuliez T, Domerego R (2003) L'Apitherapie. Bruxelles: Amyris SPRL
- Craig WJ. Health-promoting properties of common herbs. Am J Clin Nutr. 1999;70(3):491. doi: 10.1093/ajcn/70.3.491s. [DOI] [PubMed] [Google Scholar]
- Duh PD, Tu YY, Yen GC. Antioxidant activity of aqueous extract of harn jyur (Chyrsanthemum morifolium Ramat) LWT-Food Sci Technol. 1999;32:269–277. doi: 10.1006/fstl.1999.0548. [DOI] [Google Scholar]
- Fardet A. New hypotheses for the health-protective mechanisms of whole-grain cereals: what is beyond fibre. Nutr Res Rev. 2010;23(1):65–134. doi: 10.1017/S0954422410000041. [DOI] [PubMed] [Google Scholar]
- Friedlander AH, Yagiela JA, Paterno VI,Mahler ME (2003) The pathophysiology, medical management, and dental implications of children and young adults having attention-deficit hyperactivity disorder. Can Dental Asso J 31(9) [PubMed]
- Ghasemzadeh A, Jaafar HZE, Rahmat A. Antioxidant activities, total phenolics and flavonoids content in two varieties of Malaysia young ginger (Zingiber officinale Roscoe) Molecules. 2010;15(6):4324–4333. doi: 10.3390/molecules15064324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godevac D, Vajs V, Menkovi N, Teševi V, Jana kovi PT, Milosavljevi S. Flavonoids from flowers of Cephalaria pastricenis and their antiradical activity. J Serbian Chem Society. 2004;69(11):883–886. doi: 10.2298/JSC0411883G. [DOI] [Google Scholar]
- Grice HC. Safety evaluation of butylated hydroxyanisole from the perspective of effects on forestomach and oesophageal squamous epithelium. Food Chem Toxicol. 1988;26(8):717–723. doi: 10.1016/0278-6915(88)90072-5. [DOI] [PubMed] [Google Scholar]
- Hall CA. Structure-Activities of natural antioxidants. In: Cuppett SL, Aruoma OI, editors. Antioxidant Methodology. In vivo and in vitro. Concepts. Champaign: AOAC Press; 1997. [Google Scholar]
- Halliwell B, Gutteridge JM. Free radicals in biology and medicine. Oxford: Oxford University Press; 1999. [Google Scholar]
- Harborne JB, Williams CA. Advances in flavonoid research since 1992. Phytochemistry. 2000;55(6):481–504. doi: 10.1016/S0031-9422(00)00235-1. [DOI] [PubMed] [Google Scholar]
- Harish R, Shivanandappa T. Antioxidant activity and hepatoprotective potential of Phyllanthus niruri. Food Chem. 2006;95(2):180–185. doi: 10.1016/j.foodchem.2004.11.049. [DOI] [Google Scholar]
- Hassimotto NMA, Genovese MI, Lajolo FM. Antioxidant activity of dietary fruits, vegetables, and commercial frozen fruit pulps. J Agric Food Chem. 2005;53(8):2928–2935. doi: 10.1021/jf047894h. [DOI] [PubMed] [Google Scholar]
- Kahkonen MP, Hopia AI, Vuorela HJ, Rauha JP, Pihlaja K, Kujala TS, Heinonen M. Antioxidant activity of plant extracts containing phenolic compounds. J Agric Food Chem. 1999;47(10):3954–3962. doi: 10.1021/jf990146l. [DOI] [PubMed] [Google Scholar]
- Kast RE. Borage oil reduction of rheumatoid arthritis activity may be mediated by increased cAMP that suppresses tumor necrosis factor-alpha. Int Immunopharmacol. 2001;1(12):2197–2199. doi: 10.1016/S1567-5769(01)00146-1. [DOI] [PubMed] [Google Scholar]
- Klich R. Behavior disorders in childhood and their therapy. Die Medizinische Welt. 1975;26(25):1251. [PubMed] [Google Scholar]
- Korver O (1998) Functional foods: the food industry and functional foods: some European perspectives. Functional Foods for Disease Prevention: II Medicinal Plants and Other Foods American Chemical Society, Washington, DC:22–25
- Lako J, Trenerry VC, Wahlqvist M, Wattanapenpaiboon N, Sotheeswaran S, Premier R. Phytochemical flavonols, carotenoids and the antioxidant properties of a wide selection of Fijian fruit, vegetables and other readily available foods. Food Chem. 2007;101(4):1727–1741. doi: 10.1016/j.foodchem.2006.01.031. [DOI] [Google Scholar]
- Lee JH, Park JC, Choi JS. The antioxidant activity of Ecklonia stolonifera. Arch Pharma Res. 1996;19(3):223–227. doi: 10.1007/BF02976894. [DOI] [Google Scholar]
- Liu RH. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am J Clin Nutr. 2003;78(3):517S. doi: 10.1093/ajcn/78.3.517S. [DOI] [PubMed] [Google Scholar]
- Liu M, Li XQ, Weber C, Lee CY, Brown J, Liu RH. Antioxidant and antiproliferative activities of raspberries. J Agri Food Chem. 2002;50(10):2926–2930. doi: 10.1021/jf0111209. [DOI] [PubMed] [Google Scholar]
- Lugasi A, Hóvári J (2003) Antioxidant properties of commercial alcoholic and nonalcoholic beverages. Molecular Research and Food Research 47(2):79–86 [DOI] [PubMed]
- Matthaus B. Antioxidant activity of extracts obtained from residues of different oilseeds. J Agric Food Chem. 2002;50(12):3444–3452. doi: 10.1021/jf011440s. [DOI] [PubMed] [Google Scholar]
- Medoua GN, Egal AA, Oldewage-Theron WH. Nutritional value and antioxidant capacity of lunch meals consumed by elderly people of Sharpeville, South Africa. Food Chem. 2009;115(1):260–264. doi: 10.1016/j.foodchem.2008.12.007. [DOI] [Google Scholar]
- Oser BL. Hawk's physiological chemistry. New Delhi: McGraw-Hill; 1965. [Google Scholar]
- Oyaizu M. Studies on product of browning reaction produced from glucose amine. Japanese J Nutr. 1986;44:307–315. doi: 10.5264/eiyogakuzashi.44.307. [DOI] [Google Scholar]
- Podsedek A. Natural antioxidants and antioxidant capacity of Brassica vegetables: a review. LWT-Food Sci Technol. 2007;40(1):1–11. doi: 10.1016/j.lwt.2005.07.023. [DOI] [Google Scholar]
- Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of Vitamin E. Anal Biochem. 1999;269:337–341. doi: 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]
- Ranganna S (1986) Handbook of analysis and quality control for fruit and vegetable products. Tata McGraw-Hill
- Sachindra N, Airanthi MKWA, Hosokawa M, Miyashita K. Radical scavenging and singlet oxygen quenching activity of extracts from Indian seaweeds. J Food Sci Technol. 2010;47(1):94–99. doi: 10.1007/s13197-010-0022-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafaghi B, Naderi N, Tahmasb L, Kamalinjad M. Anxiolytic effect of Echium amoenum in mice. Iranian J Pharma Res. 2002;1:37–41. [Google Scholar]
- Siddhuraju P, Mohan PS, Becker K. Studies on the antioxidant activity of Indian Laburnum (Cassia fistula L.): a preliminary assessment of crude extracts from stem bark, leaves, flowers and fruit pulp. Food Chem. 2002;79(1):61–67. doi: 10.1016/S0308-8146(02)00179-6. [DOI] [Google Scholar]
- Sun T, Ho CT. Antioxidant activities of buckwheat extracts. Food Chem. 2005;90(4):743–749. doi: 10.1016/j.foodchem.2004.04.035. [DOI] [Google Scholar]
- Turkmen N, Sari F, Velioglu YS. Effects of extraction solvents on concentration and antioxidant activity of black and black mate tea polyphenols determined by ferrous tartrate and Folin-Ciocalteu methods. Food Chem. 2006;99(4):835–841. doi: 10.1016/j.foodchem.2005.08.034. [DOI] [Google Scholar]
- Velioglu YS, Mazza G, Gao L, Oomah BD. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J Agri Food Chem. 1998;46(10):4113–4117. doi: 10.1021/jf9801973. [DOI] [Google Scholar]
- Wojdylo A, Oszmianski J, Czemerys R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007;105(3):940–949. doi: 10.1016/j.foodchem.2007.04.038. [DOI] [Google Scholar]
- Wong SY. Colorimetrie determination of iron and haemoglobin in blood. J Biol Chem. 1928;77:409–412. [Google Scholar]
- Wretensjo I, Svensson L, Christie WW. Gas chromatography -mass spectrometric identification of the fatty acids in borage oil using the picolinyl ester derivatives. J Chromatography. 1990;521(1):89–97. doi: 10.1016/0021-9673(90)85067-6. [DOI] [Google Scholar]
- Wright LP, Mphangwe NIK, Nyirenda HE, Apostolides Z. Analysis of caffeine and flavan-3-ol composition in the fresh leaf of Camellia sinesis for predicting the quality of the black tea produced in Central and Southern Africa. J Sci Food Agric. 2000;80(13):1823–1830. doi: 10.1002/1097-0010(200010)80:13<1823::AID-JSFA702>3.0.CO;2-E. [DOI] [Google Scholar]
- Yen GC, Duh PD, Tsai CL. Relationship between antioxidant activity and maturity of peanut hulls. J Agri Food Chem. 1993;41(1):67–70. doi: 10.1021/jf00025a015. [DOI] [Google Scholar]
- Yu BP. Cellular defenses against damage from reactive oxygen species. Physiol Rev. 1995;75(1):236. doi: 10.1152/physrev.1994.74.1.139. [DOI] [PubMed] [Google Scholar]
- Zhou K, Yu L. Effects of extraction solvent on wheat bran antioxidant activity estimation. LWT-Food Sci Technol. 2004;37(7):717–721. doi: 10.1016/j.lwt.2004.02.008. [DOI] [Google Scholar]