Abstract
Soy protein is increasingly used in extended meat products and dairy type products due to the presence of high quality proteins with excellent functional properties. However, it has been shown to inhibit iron bioavailability because of phytic acid present in the protein. This present study investigated the effects of dephytinise from soy protein isolate (SPI) on iron binding capacity and degree of hydrolysis. Also the effects of enzyme type and degree of hydrolysis on iron binding capacity were studied. It was demonstrated that phytase and anion exchange resin could remove effectively the phytate from SPI. The dephytinise would decrease the degree of hydrolysis of SPI. The enzyme type and degree of hydrolysis influenced significantly the iron binding capacity of the hydrolysate. Flavourzyme might be the best choice for producing peptides with iron binding capacity from SPI and middle degree of hydrolysis would be benefitable to this process.
Keywords: Iron binding capacity, Soy protein isolate, Hydrolysis, Phytate
Introduction
Iron is considered to be one of the essential minerals required by plants, animals and humans. It is essential components of cytochromes, hemoglobin, myoglobin and some enzymes. Transport of oxygen and electrons is one of its major functions. It has been reported nearly one-fifth of the world population has iron deficiency anemia and nutritional iron deficiency was identified as one of the 10 leading risk factors for disease, disability and death in the world today (WHO 2002). Therefore, fortification with iron in many foods, such as milk or dairy products, soy sauce and wheat flour, has been considered as a potential approach to prevent iron deficiency in humans. However, some problems should be paid attention to iron fortification, including variable bioavailability, organoleptic defects, formation of sediments and the effect of iron on lipid oxidation (Sugiarto et al. 2009).
Enzymatic hydrolysis, which influences molecular size, hydrophobicity and polar groups of the hydrolysate, can improve the functional properties of protein. The degree of hydrolysis (DH) has the positive or negative effects on the functional proterties of hydrolysate. Salmon byproduct hydrolysate has an excellent solubility at high DH, but its emulsifying capacity and emulsion stability were noticeably greater at low DH (Gbogouri et al. 2004). Casein phosphopeptides (CPP) has been shown to improve the availability of calcium, zinc and iron (Yeung et al. 2002). Besides CPP, in recent years some small peptides from other sources have been reported to posses high iron binding capacity or increased bioavailability of iron, including whey protein (Lee and Song 2009; Gabriel et al. 2004; Kim et al. 2007a, b), porcine blood plasma protein (Lee and Song 2009), shrimp process by-products protein (Huang et al. 2011), fish protein (Deng et al. 2008) and muscle tissue protein (Storcksdieck et al. 2007). Soybean can be considered the largest potential source of protein for humans since it could account for one-third of the worldwide protein requirement. It is well known that soybean protein or peptides have various physiological activities, such as insulin resistance and weight loss in obesity (Volgarev et al. 1989), cholesterol-lowering effect (Carroll 1991; Iritani et al. 1997), reduction of blood pressure (Hermansen et al. 2001), antioxidative (Hou et al. 2011), anticancer (Kim et al. 2000), hypocholesterolemia (Zhong et al. 2007), angiotensin I-converting enzyme (ACE) inhibitory activity (Kuba et al. 2005), immunomodulation (Kong et al. 2008), hypotriglyceridemic activity (Kagawa et al. 1996), regulating food intake (Kwon et al. 2010), anti-inflammatoration (Dia et al. 2009) and mineral binding activity (Bao et al. 2007). However, soy protein products are known to contain appreciable quantities of phytate, which is an important inhibitor of mineral absorption. It has been reported that the bioavailability of iron in soy protein isolate (SPI) was relatively less than that of cow milk protein or human milk protein. This low iron bioavailability was mainly attributed to high phytic acid content in SPI. Phytate bound to soy protein and interfered with the dietary iron absorption in the small intestine by forming a complex and insolubilizing it. Dephytinise from SPI will enhance the bioavailability of iron (Hurrell et al. 1992). Therefore, many researchers have made efforts to dephytinise from soybean proteins using acidic and alkaline reagents, cations, ethylenediamine tetraacetic acid (EDTA), ultrafiltration and anion exchange resins (Kumagai et al. 1998; Okubo et al. 1975; Ford et al. 1978; Omosaiye and Cheryan 1979; Brooks and Morr 1982; Kumagai et al. 2002). A lot of peptides with mineral binding ability have been isolated and characterized from marine protein hydrolysate (Jung and Kim 2007; Jung et al. 2005, 2006), whey protein (Rui 2009), yolk phosvitin (Jiang and Mine 2001) and soy protein (Bao et al. 2007). However, there is a little information regarding iron binding peptide from dephytatinised SPI and its iron binding properties as affected by DH and enzyme used. Therefore, this study aimed to produce iron delivering peptides containing low phytic acid from SPI using several proteinases and to study iron binding activity of the hydrolysate at different DHs.
Materials and methods
Materials
SPI with protein content of 93.8% was obtained from Huayuan Bean Product Co. (Hangzhou, China). Two proteases including pepsin (250U/mg) and trypsin (2,500U/mg) were obtained from Sigma Chemical Co. (St. Louis, MO), and four proteases including Alcalase (150U/mg), Neutrase (200U/mg), Flavourzyme (50U/mg), and Protamex (120U/mg) were purchased from Denyken Co. (Shanghai, China). The phytase (50U/mg) from Aspergillus niger was obtained from Keyuan Biotechnol Co. (Shanghai, China). All other reagents were of the highest grade available commercially.
Dephytatinise of SPI
Three methods, including phytase, resin and acid-organic solvent treatments, were used to dephytinise from SPI.
Phytase treatment
10.0 g SPI was dissolved in 200 ml distilled water and the solution was adjusted to pH 5.5 with 2 N HCl. Phytase (4,000U) was added into the protein solution. Enzymatic hydrolysis was then performed on these solutions at 50 °C for 4 h. The pH was adjusted to 5.5 every 15 min during the hydrolysis. Following hydrolysis, the aliquots were heated in a boiling water bath for 5 min to deactivate phytase and then cooled to room temperature and adjusted to pH 4.5. Subsequently, the solutions were kept for 2 h at 4 °C and then centrifuged at 8,000 g for 20 min, and the precipitate was rewashed twice by distilled water at pH4.5. The precipitate was collected and lyophilized.
Anion exchange resin treatment
The resin with a 2-hydroxyethyl dimethyl ammonium group (D101) was used to dephytinise according to the method of Kumagai et al. (2002) with some modifications. In simply, the anion exchange resin was washed successively with 1.0 N HCl, distilled water, 1.0 N NaOH, distilled water and 0.05 M pH 7.4 Tris–HCl buffer. The soy proetin isolate (0.5%, w/v) was disperated in 0.05 M Tris–HCl buffer-resin solution and stirred at 4 °C for 4 h. Then the mixture was filtered through duplex cotton cloth and the filtrate was dialyzed overnight against distilled water at 4 °C. The contents were collected and lyophylized.
Acid-organic solvent treatment
10.0 g SPI was suspended in 500 ml acid mixture solution (HCl/ethanol/water = 4:90:6, v/v) and refluxed for 60 min at 75 °C. Then the mixture was centrifuged at 8,000 g for 20 min, and the precipitate was rewashed twice by distilled water at pH4.5. The precipitate was collected and lyophilized.
Hydrolysis of dephysinised SPI
Effects of enzyme type
The dephytinised SPI was suspended at 2.0% (w/v) level in 20 mM Tris–HCl or KCl-HCl buffer at the optimal conditions of pH and temperature for each enzyme. The optimal pH of trypsin, pepsin, Protemax, Flavorzyme, Neutrase and Alcalase were 8.0, 2.0, 7.5, 7.1, 7.1 and 8.0, respectively. The optimal temperature of trypsin and pepsin was 37 °C. The optimal temperature of Protemax, Flavorzyme, Neutrase and Alcalase was 50 °C. Then six powder proteases (0.02%, w/v) were added respectively. Then the mixtures were stirred at optimum temperature for 120 min. Following hydrolysis, the solutions were heated in a boiling water bath for 5 min to deactivate protease and afterward cooled to room temperature. Subsequently, the solutions were centrifuged at 8,000 g for 20 min, and the supernatant was used for determination of iron binding capacity and degree of hydrolysis.
Effects of degree of hydrolysis
A 2.0% (w/v) dephytinised SPI was suspended in 20 mM Tris–HCl buffer at pH 7.1. Then the Flavourzyme was added at 0.02% (w/v) level. The protein solution was stirred at 50 °C and 10 ml sample was collected at 20 min interval to determine the iron-binding capacity and degree of hydrolysis.
Determination of iron binding capacity
The iron binding capacity was determined according to the method of Decker and Welch (1990) with some modifications. One milliliter of sample solution was mixed with 3.0 ml of distilled water. The mixture was then reacted with 0.1 ml of 1 mM FeCl2 and 0.2 ml of 5 mM 3-(2-pyridyl)-5,6-bis (4- phenyl-sulfonic acid) -1,2,4-triazine (ferrozine) for 20 min at room temperature. The absorbance was read at 562 nm. The control was prepared in the same manner except that distilled water was used instead of the sample. EDTA was used as positive control. The iron binding capacity was expressed with equivalent EDTA per gram protein.
Determination of degree of hydrolysis
The DH of SPI hydrolysates was determined according to the method of Pericin et al. (2009) with some modifications. A 0.5 ml 20% (w/w) trichloroacetic acid (TCA) was added to an equal volume of hydrolysates. Then the mixture was kept for 30 min at 4 °C. Thereafter, the mixture was centrifuged at 12,000 g for 10 min. The peptide content in the supernatant and the original protein content before hydrolysis were determined by method of Lowry et al. (1951), using bovine serum albumin (BSA) as the standard protein. The DH was calculated as the ratio of TCA-soluble peptide to total protein before hydrolysis in the mixture, expressed as a percentage.
Determination of phytate content
A 0.200 g SPI was extracted by 10 ml of 0.5 N HCl for 60 min at room temperature. Subsequently, the mixture was centrifuged for 15 min at 5,000 g. The supernatants were used to measure the phytate content by reverse phase high performance liquid chromatography (RP-HPLC) according to Dost and Tokul (2006) method.
Molecular weight distribution
The molecular weight (MW) distributions of the hydrolysates were determined by gel permeation chromatography (GPC) using an HPLC system (Shimadzu LC20A, Toyko, Japan). The GPC columns, Cosmosil 5Diol-120-II (7.5 × 300 mm) (Nacalai Tesque, Toyko, Japan), were connected, and the hydrolysates were chromatographed and monitored at 220 nm at room temperature. The MW standards were BSA (66,700 Da), cytochrome C (12,400 Da), aprotinin (6,500 Da), and vitamin B12 (1,355 Da).
Statistical analysis
All the experiments were carried out in triplicate, and the data obtained were subjected to statistical analysis using ANOVA; the least significant difference (LSD) with a confidence interval of 95% was used to compare the means.
Results and discussion
Dephytatinise of SPI
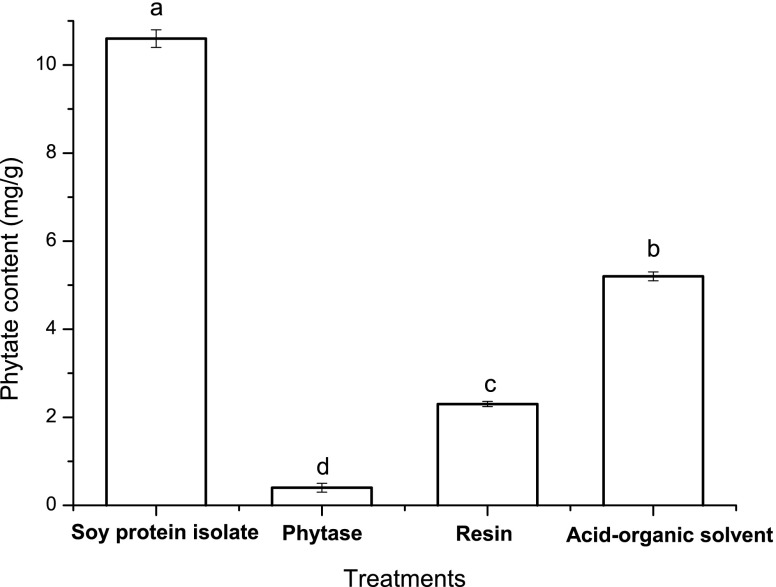
In this study, we compared the effects of phytase, anion exchange resin and acid-ethanol treatments to dephytatinise from SPI. The result (Fig. 1) showed that phytase treatment was the most effective for dephytinise from SPI and 95% of phytate was hydrolysised by the enzyme. Anion exchange resin was also effective for dephytatinise from SPI, which eliminated about 80% phytate in the protein. The resin can be used for eliminating phytate from soy protein in commercial purpose because of its operating convenience, relative inexpensive and repeatable.
Fig. 1.
Dephytatinise from soy proetin isolate by phytase, anion exchange resin and acid-organic solvent treatments. Each observation is a mean of three replicate experiments (n = 3) and the values with different lowercase differ significantly at p < 0.05
Effects of enzyme type and DH on iron binding capacity
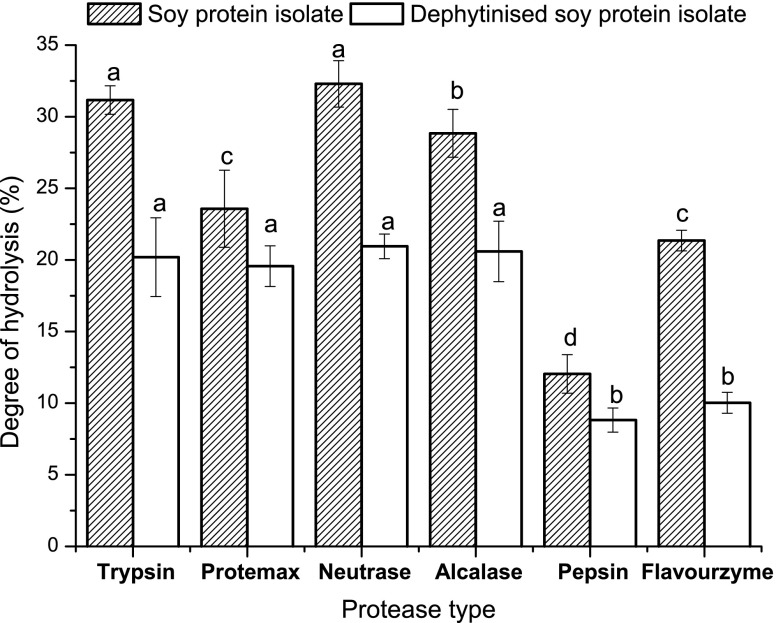
With the same protein substrate (2.0%, w/v) and the same amount of enzyme (0.02%, w/v), the Alcalase, typsin and Neutrase showed a higher DH than did Flavourzyme and pepsin (Fig. 2). Also, the lower DH was observed with all enzymes after dephytatinise from the SPI. Generally, alkaline proteases, including Alcalase and typsin, exhibited higher activities than did acid or neutral proteases such as Flavourzyme (Rebeca et al. 1991). Hydrolysis can increase the iron binding capacity of SPI (Table 1). However, the iron binding capacity of the hydrolysates was not proportional to the DH. At 2.0% SPI and 0.02% enzyme addition, the Flavourzyme showed a lower DH than did prometrax, typsin, and neutrase. However, the hydrolysate of Flavourzyme showed a higher iron binding capacity than that of Protemax, typsin, or Neutrase. Due the higher iron binding capacity of hydrolysate, the Flavourzyme was used in the further studies.
Fig. 2.
The degree of hydrolysis (DH) of soy proetin isolate and dephytatinised soy proetin isolate under different proteases hydrolysis. Each observation is a mean of three replicate experiments (n = 3) and the values with different lowercase differ significantly at p < 0.05
Table 1.
Effects of enzyme type on iron binding capacity and degree of hydrolysis (DH) of dephytatinised soy proetin isolate
| Enzyme | Iron binding capacity (mg EDTA/g-protein) | DH (%) |
|---|---|---|
| Control | 0.71 ± 0.10 e | ____ |
| Trypsin | 2.85 ± 0.11 c | 20.19 ± 2.75 a |
| Protemax | 3.51 ± 0.04 b | 19.56 ± 1.42 a |
| Neutrase | 2.68 ± 0.13 c | 20.95 ± 0.86 a |
| Alcalase | 0.77 ± 0.16 d | 20.59 ± 2.11 a |
| Pepsin | 0.97 ± 0.12 d | 8.82 ± 0.84 c |
| Flavorzyme | 3.76 ± 0.14 a | 10.02 ± 0.73 b |
Values are means ± SD of three determinations and the values with the same letters denote no significant difference (p < 0.05). EDTA means ethylenediamine tetraacetic acid
The iron binding capacity of hydylosate was affected significantly by the DH during Flavourzyme hydrolysis (Table 2). The iron binding capacity of hydrolysate reached the highest of 3.87 mg EDTA/g-protein at DH of 6.79% and then decreased to 2.22 mg EDTA/g-protein with DH of 11.17%. Many researchers have reported the relationship between DH and antioxidative activity or metal chelating activity using different proteases hydrolysis of muscle or plant proteins. Klompong et al. (2007) reported that the Alcalase hydrolysate of yellow stripe trevally muscle protein had higher 1,1-Diphenyl-2-picrylhydrazyl (DPPH) radical-scavenging activity at lower DH (5%), but it had higher metal chelating activity at higher DH (25%). The similar results also were reported by Jamdar et al. (2010) using Alcalase 2.4 L to hydrolysis peanut protein. However, Liu et al. (2010) reported that porcine plasma protein Alcalase hydrolysate had higher DPPH radical-scavenging activity and metal chelating activity at higher DH (17.6%). Khantaphant et al. (2011) reported that the relationship between DH and antioxidative or metal chelating activity was depended on enzyme type. The protein hydrolysate from brown stripe red snapper muscle had higher metal chelating activity at higher DH when using pyloric caeca protease from brown stripe red snapper; however, it had higher metal chelating activity when using Alcalase and Flavourzyme at lower DH. So, the effect of DH on the iron binding capacity was complex and depended by the protease type and protein source. Chen et al. (1998) also reported that the size and sequence of amino acids in the resulting peptides most likely determine the antioxidant activity of protein hydrolysates.
Table 2.
Effects of degree of hydrolysis (DH) on iron binding capacity of dephytatinised soy protein isolate Flavourzyme hydrolysate
| DH (%) | Iron binding capacity (mg EDTA/g-protein) |
|---|---|
| 0 | 0.71 ± 0.10 e |
| 4.99 | 2.09 ± 0.21 d |
| 6.79 | 3.87 ± 0.14 a |
| 9.89 | 3.02 ± 0.35 b |
| 10.87 | 2.67 ± 0.09 c |
| 11.17 | 2.22 ± 0.28 d |
Values are means ± SD of three determinations and the values with the same letters denote no significant difference (p < 0.05). EDTA means ethylenediamine tetraacetic acid
Molecular weight distribution
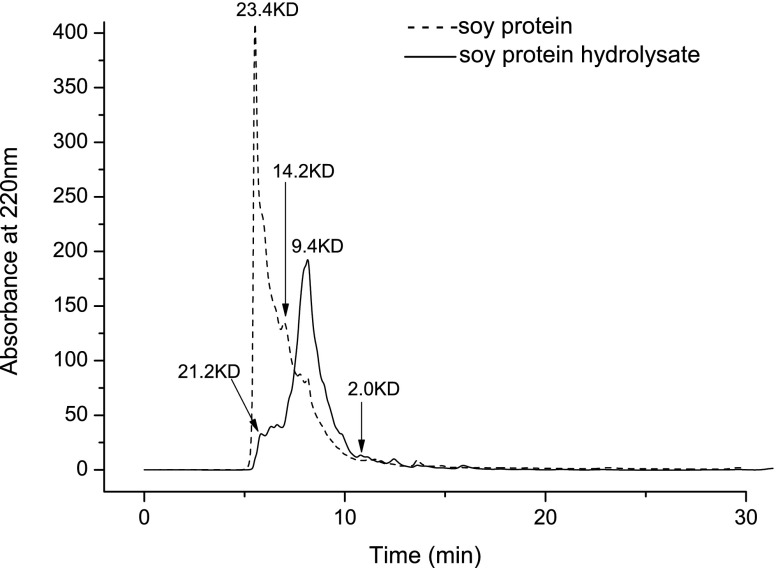
To study the molecular weight distributions of SPI hydrolysate, the gel permeation chromatography (GPC) using RP-HPLC system was used. According to the linear regression of Log(MW) and elution time, the chromatographic data (Fig. 3) indicated that the molecular mass of the main peaks of SPI hydrolysate was found to be mostly distributed among 2–10 kDa. However, the molecular mass of SPI was found to focus on 10–23 kDa. Most of the reported peptides exhibiting biological activity, including antioxidative or metal chelating activity, were those with low molecular weights (Rajapakse et al. 2005) and were usually more effective in intestine (Webb 1986). The relationship between molecular weight and iron binding activity, as well as purification and characterization of peptides with high iron binding capacity should be studied furthermore.
Fig. 3.
The molecular weight distribution of soy proetin isolate and its hydrolysate with Flavourzyme at degree of hydrolysis (DH) of 6.79%
Conclusions
There are numerous reports on bioactive peptides from soy protein by exogenous enzymatic hydrolysis or fermentation. There are no reports to date regarding the effects of DH and enzyme type on iron binding capacity of peptides from dephytatinised SPI. It was investigated to the effects of phytase, resin, and acid-organic solvent treatments on dephytatinise. In addition, the effect of dephytatinise on DH was also tested and the result showed that the DH of low-phytate protein was lower rather than that of original soy protein. It was also shown that Flavourzyme might be more suitable for producing peptides with higher iron binding capacity from SPI. The hydrolysate with moderate DH had higher iron binding capacity than did the hydrolysate with higher or lower DH. The peptides from SPI have the potential to be a good dietary supplement for prevention of iron deficiency anemia and nutritional iron deficiency.
Acknowledgement
This work was granted by Zhejiang Provincial Natural Science Foundation of China under Grant No. Y 3110278.
References
- Bao XL, Song M, Zhang J, Yang C, Guo ST. Calcium-binding ability of soy protein hydrolysates. Chin Chem Lett. 2007;18:1115–1118. doi: 10.1016/j.cclet.2007.07.032. [DOI] [Google Scholar]
- Brooks JR, Morr CV. Phytate removal from soy protein isolates using ion exchange processing treatments. J Food Sci. 1982;47:1280–1282. doi: 10.1111/j.1365-2621.1982.tb07666.x. [DOI] [Google Scholar]
- Carroll KK. Review of clinical studies on cholesterol lowing response to soy protein. Am J Diet Assoc. 1991;91:820–827. [PubMed] [Google Scholar]
- Chen HM, Muramoto K, Yamaguchi F, Fujimoto NK. Antioxidative properties of histidine-containing peptides designed from peptide fragment found in digests of a soybean protein. J Agric Food Chem. 1998;46:49–53. doi: 10.1021/jf970649w. [DOI] [PubMed] [Google Scholar]
- Decker EA, Welch B. Role of ferritin as a lipid oxidation catalyst in muscle food. J Agric Food Chem. 1990;38:674–677. doi: 10.1021/jf00093a019. [DOI] [Google Scholar]
- Deng SG, Huo JC, Xie C. Preparation by enzymolysis and bioactivity of iron complex of fish protein hydrolysate (Fe-FPH) from low value fish. Chin J Ocean. 2008;26:300–306. doi: 10.1007/s00343-008-0300-4. [DOI] [Google Scholar]
- Dia VP, Wang W, Oh VL, Lumen BO, Meji EG. Isolation, purification and characterisation of lunasin from defatted soybean flour and in vitro evaluation of its anti-inflammatory activity. Food Chem. 2009;114:108–115. doi: 10.1016/j.foodchem.2008.09.023. [DOI] [Google Scholar]
- Dost K, Tokul O. Determination of phytic acid in wheat and wheat products by reverse phase high performance liquid chromatography. Anal Chimica Acta. 2006;558:22–27. doi: 10.1016/j.aca.2005.11.035. [DOI] [Google Scholar]
- Ford JR, Mustakas GC, Schmdutz RD. Phytic acid removal from soybeans by a lipid protein concentrate process. J Am Oil Chem Soc. 1978;55:371–374. doi: 10.1007/BF02911893. [DOI] [Google Scholar]
- Gabriel ER, Erick B, Muriel S. Iron availability from whey protein hydrogels: an in vitro study. J Agric Food Chem. 2004;52:8137–8143. doi: 10.1021/jf040286h. [DOI] [PubMed] [Google Scholar]
- Gbogouri GA, Linder M, Fanni J, Parmentier M. Influence of hydrolysis degree on the functional properties of salmon byproduct hydrolysates. J Food Sci. 2004;69:615–622. doi: 10.1111/j.1365-2621.2004.tb09909.x. [DOI] [Google Scholar]
- Hermansen K, Sondergaard M, Hoie L, Carstensen M, Brock B. Beneficial effects of a soy-based dietary supplement on lipid levels and cardiovascular risk markers in type 2 diabetic subjects. Diabetes Care. 2001;24:228–233. doi: 10.2337/diacare.24.2.228. [DOI] [PubMed] [Google Scholar]
- Hou X, Wang J, Zhang D (2011) Optimization of debittering of soybean antioxidant hydrolysates with β-cyclodextrins using response surface methodology. J Food Sci Technol. doi:10.1007/s13197-011-0358-4 [DOI] [PMC free article] [PubMed]
- Huang GR, Ren ZY, Jiang JX. Separation of iron-binding peptides from shrimp processing by-products hydrolysates. Food Bioprocess Technol. 2011;4:1527–1532. doi: 10.1007/s11947-010-0416-3. [DOI] [Google Scholar]
- Hurrell RF, Juillerat MA, Reddy MB, Lynch SR, Dassenko SA, Cook JD. Soy protein, phytate, and iron absorption in humans. Am J Clin Nutr. 1992;56:573–578. doi: 10.1093/ajcn/56.3.573. [DOI] [PubMed] [Google Scholar]
- Iritani N, Sugimoto T, Fukuda H. Dietary soybean protein increases insulin receptor gene expression in male Wistar fatty rats when dietary polyunsaturated fatty acid level is low. J Nutr. 1997;127:1077–83. doi: 10.1093/jn/127.6.1077. [DOI] [PubMed] [Google Scholar]
- Jamdar SN, Rajalakshmi V, Pednekar MD, Juan F, Yardi V, Sharma A. Influence of degree of hydrolysis on functional properties, antioxidant activity and ACE inhibitory activity of peanut protein hydrolysate. Food Chem. 2010;121:178–184. doi: 10.1016/j.foodchem.2009.12.027. [DOI] [Google Scholar]
- Jiang B, Mine Y. Phosphopeptides derived from hen egg yolk phosvitin: effect of molecular size on the calcium-binding properties. Biosci Biotech Bioch. 2001;65:1187–1190. doi: 10.1271/bbb.65.1187. [DOI] [PubMed] [Google Scholar]
- Jung WK, Kim SK. Calcium-binding peptide derived from pepsinolytic hydrolysates of hoki (Johnius belengerii) frame. Eur Food Res Technol. 2007;224:763–767. doi: 10.1007/s00217-006-0371-4. [DOI] [Google Scholar]
- Jung WK, Park PJ, Byun HG, Moon SH, Kim SK. Preparation of hoki (Johnius belengerii) bone oligophosphopeptide with a high affinity to calcium by carnivorous intestine crude proteinase. Food Chem. 2005;91:333–340. doi: 10.1016/j.foodchem.2004.06.016. [DOI] [Google Scholar]
- Jung WK, Karawita R, Heo SJ, Lee BJ, Kim SK, Jeon YJ. Recovery of a novel Ca-binding peptide from Alaska Pollack (Theragra chalcogramma) backbone by pepsinolytic hydrolysis. Process Biochem. 2006;41:2097–2100. doi: 10.1016/j.procbio.2006.05.008. [DOI] [Google Scholar]
- Kagawa K, Matsutaka H, Fukuhama C, Watanabe Y, Fujino H. Globin digest, acidic protease hydrolysate, inhibits dietary hypertriglyceridemia and Val-Val-Tyr-Pro, one of its constituents, possesses most superior effect. Life Sci. 1996;58:1745–1755. doi: 10.1016/0024-3205(96)00156-7. [DOI] [PubMed] [Google Scholar]
- Khantaphant S, Benjakul S, Kishimura H. Antioxidative and ACE inhibitory activities of protein hydrolysates from the muscle of brown stripe red snapper prepared using pyloric caeca and commercial proteases. Process Biochem. 2011;46:318–327. doi: 10.1016/j.procbio.2010.09.005. [DOI] [Google Scholar]
- Kim SE, Kim HH, Kim JY, Kang YI, Woo HJ, Lee HJ. Anticancer activity of hydrophobic peptides from soy proteins. Biofactors. 2000;12:151–155. doi: 10.1002/biof.5520120124. [DOI] [PubMed] [Google Scholar]
- Kim SB, Seo IS, Khan MA, Ki KS, Nam MS, Kim HS. Separation of iron-binding protein from whey through enzymatic hydrolysis. Int Dairy J. 2007;17:625–631. doi: 10.1016/j.idairyj.2006.09.001. [DOI] [Google Scholar]
- Kim SB, Seo IS, Khan MA, Ki KS, Lee WS, Lee HJ, Shin HS, Kim HS. Enzymatic hydrolysis of heated whey: iron-binding ability of peptides and antigenic protein fractions. J Dairy Sci. 2007;90:4033–4042. doi: 10.3168/jds.2007-0228. [DOI] [PubMed] [Google Scholar]
- Klompong V, Benjakul S, Kantachote D, Shahidi F. Antioxidative activity and functional properties of protein hydrolysate of yellow stripe trevally (Selaroides leptolepis) as influenced by the degree of hydrolysis and enzyme type. Food Chem. 2007;102:1317–1327. doi: 10.1016/j.foodchem.2006.07.016. [DOI] [Google Scholar]
- Kong XZ, Guo MM, Hua YF. Enzymatic preparation of immunomodulating hydrolysates from soy proteins. Bioresource Technol. 2008;99:8873–8879. doi: 10.1016/j.biortech.2008.04.056. [DOI] [PubMed] [Google Scholar]
- Kuba M, Tana C, Tawata S, Yasuda M. Production of angiotensin I-converting enzyme inhibitory peptides from soybean protein with Monascus purpureus acid proteinase. Process Biochem. 2005;40:2191–2196. doi: 10.1016/j.procbio.2004.08.010. [DOI] [Google Scholar]
- Kumagai H, Shizawa Y, Sakurai H, Kumagai H. Influence of phytate removal and structural modification on the calcium-binding properties of soybean globulins. Biosci Biotech Bioch. 1998;62:341–346. doi: 10.1271/bbb.62.341. [DOI] [PubMed] [Google Scholar]
- Kumagai H, Ishida S, Koizumi A, Sakurai H, Kumagai H. Preparation of phytate-removed deamidated soybean globulins by ion exchangers and characterization of their calcium-binding ability. J Agric Food Chem. 2002;50:172–176. doi: 10.1021/jf011011u. [DOI] [PubMed] [Google Scholar]
- Kwon DY, Daily JW, Kim HJ, Park S. Antidiabetic effects of fermented soybean products on type-2 diabetes. Nutr Res. 2010;30:1–13. doi: 10.1016/j.nutres.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Lee SH, Song KB. Purification of an iron-binding nona-peptide from hydrolysates of porcine blood plasma protein. Process Biochem. 2009;44:378–381. doi: 10.1016/j.procbio.2008.12.001. [DOI] [Google Scholar]
- Liu Q, Kong BH, XiongYL XXF. Antioxidant activity and functional properties of porcine plasma protein hydrolysate as influenced by the degree of hydrolysis. Food Chem. 2010;118:403–410. doi: 10.1016/j.foodchem.2009.05.013. [DOI] [Google Scholar]
- Lowry OH, Rosenbrough NJ, Fair AL, Randall RJ. Protein measurement with the Folin-phenol reagents. J Bio Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Okubo K, Waldrop AB, Iacobucci GA, Myers DV. Preparation of low-phytate soybean protein isolate and concentrate by ultrafiltration. Cereal Chem. 1975;52:263–271. [Google Scholar]
- Omosaiye O, Cheryan M. Low-phytate, full-fat soy protein product by ultrafiltration of aqueous extracts of whole soybeans. Cereal Chem. 1979;56:58–62. [Google Scholar]
- Pericin D, Radulovic-Popovic L, Vastag Z, Madarev-Popovic S, Trivic S. Enzymatic hydrolysis of protein isolate from hull-less pumpkin oil cake: application of response surface methodology. Food Chem. 2009;115:753–757. doi: 10.1016/j.foodchem.2008.12.040. [DOI] [Google Scholar]
- Rajapakse N, Mendis E, Byun HG, Kim SK. Purification and in vitro antioxidative effects of giant squid muscle peptides on free radical-mediated oxidative systems. J Nutr Biochem. 2005;16:562–569. doi: 10.1016/j.jnutbio.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Rebeca BD, Pena-vera MT, Diaz-Castaneda M. Production of fish protein hydrolysates with bacteria proteases: yield and nutritional value. J Food Sci. 1991;56:309–314. doi: 10.1111/j.1365-2621.1991.tb05268.x. [DOI] [Google Scholar]
- Rui XU. Calcium binding of peptides derived from enzymatic hydrolysates of whey protein concentrate. Int J Dairy Technol. 2009;62:170–173. doi: 10.1111/j.1471-0307.2009.00477.x. [DOI] [Google Scholar]
- Storcksdieck S, Bonsmann G, Hurrell RF. Iron-binding properties, amino acid composition, and structure of muscle tissue peptides from in vitro digestion of different meat sources. J Food Sci. 2007;72:S019–029. doi: 10.1111/j.1750-3841.2006.00229.x. [DOI] [PubMed] [Google Scholar]
- Sugiarto M, Ye A, Singh H. Characterisation of binding of iron to sodium caseinate and whey protein isolate. Food Chem. 2009;114:1007–1013. doi: 10.1016/j.foodchem.2008.10.062. [DOI] [Google Scholar]
- Volgarev MN, Vysotsky VG, Meshcheryakova VA. Evaluation of isolated soy protein foods in weight reduction with obese hyocholesterolemic and normocholesterolemic obese individuals. Nutr Rep Int. 1989;39:61–72. [Google Scholar]
- Webb KE. Amino acid and peptide absorption from the gastrointestinal tract. Fed Proc. 1986;45:2268–2271. [PubMed] [Google Scholar]
- WHO (2002) The World Health Report 2002-Reducing Risks, Promoting Healthy Life. World Health Organization [DOI] [PubMed]
- Yeung AC, Glahn RP, Miller DD. Effects of iron source on iron availability from casein and casein phosphopeptides. J Food Sci. 2002;67:1271–1275. doi: 10.1111/j.1365-2621.2002.tb09489.x. [DOI] [Google Scholar]
- Zhong F, Liu JM, Ma JG, Shoemaker CF. Preparation of hypocholesterol peptides from soy protein and their hypocholesterolemia effect in mice. Food Res Intl. 2007;40:661–667. doi: 10.1016/j.foodres.2006.11.011. [DOI] [Google Scholar]