Figure 2.
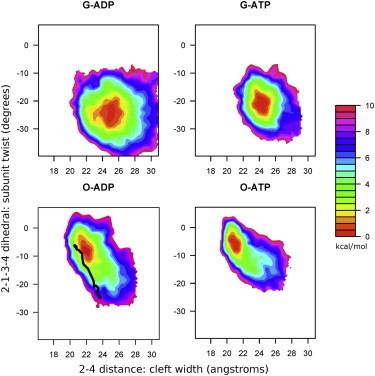
The 2D free-energy surface of the actin monomer as a function of both the dihedral twist and the nucleotide cleft width reveals that both collective variables are affected by the bound nucleotide, and that ADP-bound actin is more conformationally mobile in both the Oda and G-actin conformations. The line in the Oda-ADP panel indicates the transition path between G-actin and Oda structures, as identified using the string method with a double-well CG potential. To see this figure in color, go online.
