Abstract
Background
Weight loss is associated with bone loss, but this has not been examined in overweight premenopausal women.
Objective
The aim of this study was to assess whether overweight premenopausal women lose bone with moderate weight loss at recommended or higher than recommended calcium intakes.
Design
Overweight premenopausal women [n = 44; (±SD) age: 38 ± 6.4 y; body mass index (BMI): 27.7 ± 2.1 kg/m2] were randomly assigned to either a normal (1 g/d) or high (1.8 g/d) calcium intake during 6 mo of energy restriction [weight loss (WL) groups] or were recruited for weight maintenance at 1 g Ca/d intake. Regional bone mineral density and content were measured by dual-energy X-ray absorptiometry, and markers of bone turnover were measured before and after weight loss. True fractional calcium absorption (TFCA) was measured at baseline and during caloric restriction by using a dual-stable calcium isotope method.
Results
The WL groups lost 7.2±3.3% of initial body weight. No significant decrease in BMD or rise in bone turnover was observed with weight loss at normal or high calcium intake. The group that consumed high calcium showed a strong relation (r = 0.71) between increased femoral neck bone mineral density and increased serum 25-hydroxyvitamin D. No significant effect of weight loss on TFCA was observed, and the total calcium absorbed was adequate at 238±81 and 310±91 mg/d for the normal- and high-calcium WL groups, respectively.
Conclusion
Overweight premenopausal women do not lose bone during weight loss at the recommended calcium intake, which may be explained by sufficient amounts of absorbed calcium.
Keywords: Bone, calcium absorption, hormones, premenopausal women, weight loss
INTRODUCTION
There are a limited number of weight loss studies in premenopausal women showing either bone loss (1–4) or no change (5). A history of weight loss in premenopausal years has been associated with an increase in hip fracture risk later in life (6). In addition, a weight loss of 5–10% in postmenopausal women is associated with a decrease in bone mass of ≈1–4% (7–12) and an increase in bone resorption (7, 13). Further evidence from observational studies shows that a decrease in body weight in overweight [body mass index (BMI; in kg/m2) of 25 to 29.9] compared with obese (BMI = 30 to 40) older women results in greater bone loss (14). Compared with obese persons, overweight persons may be more susceptible to bone loss due to reduced weight bearing (9, 15, 16), diminished extraovarial estrogen synthesis due to smaller fat depots (17, 18), and reduced calcium intake due to lower energy requirements, especially during dieting (1, 5, 19). To our knowledge, there have been no controlled weight loss trials examining overweight premenopausal women to determine bone loss, nor has the influence of recommended or high calcium intake been addressed. In addition, whether caloric restriction compromises calcium absorption in premenopausal women, as has been observed in older women (20), was an important goal in the present study. Our aims were to examine bone turnover and mass in overweight premenopausal women with weight reduction at recommended or high calcium intakes and to determine the role of calcium absorption and hormonal regulators.
SUBJECTS AND METHODS
Subjects
Sixty weight-stable (>3 mo), healthy, overweight, premenopausal women were recruited to participate in a weight loss (WL) or weight maintenance (WM) intervention, beginning either in the spring or fall. Advertisement for recruitment was done in local newspapers and radio stations every 6 mo between the years 2002 and 2004. Exclusion criteria included irregular menstrual cycle, pregnancy or nursing a child within the past year, taking oral contraceptives or other medications known to influence bone metabolism, evidence of metabolic bone disease or other diseases such as thyroid disorders, immune disease, heart attack or stroke in the past 6 mo, kidney stones, diabetes, and active cancers or cancer therapy within the past 12 mo. Before the beginning of the study, the participants had to be weight stable for ≥3 mo. The participants were instructed to maintain their weight until the beginning of the study and not to change physical activity levels. The study was approved by the Rutgers University Institutional Review Board, and all study participants signed an informed consent form before entering the study.
Study design
The participants were asked to maintain their body weight and stop taking any dietary supplements for ≥4 wk before the beginning of the WL or WM intervention. During this stabilization period, the participants were given a standard daily multivitamin and mineral (MVi) supplement (Sentury-Vite Pharmavite Corp, Mission Hills, CA), containing 200 mg Ca and 400 IU vitamin D. After this 1-mo stabilization, baseline measurements were taken. Thereafter, women adhered to 6 mo of weight loss or maintenance followed by 1 additional month of weight stabilization, at which time bone density measurements were taken. Hence, with a month of stabilization before and after weight loss, the total time commitment was 8 mo. To avoid the negative influence of a low calcium diet on bone mass during dieting, all women in the present study were supplemented with calcium, at either high (1.8 g/d) or the recommended (1 g/d) levels. The group with recommended or adequate calcium intake (1 g/d) was labeled “normal” because it matched their usual intake and this label of “normal” is consistent with our previous publications (12, 20). The participants recruited for the WL group were randomly assigned in a double-blind manner to 1 of 2 interventions receiving calcium supplements: 1) 1000 mg Ca [high calcium (HiCa); WL HiCa group] or 2) 200 mg Ca [normal calcium (NLCa); WL NLCa]. The supplement was given as calcium citrate (200 mg Ca/tablet) and placebo tablets (gift from Mission Pharmacal, San Antonio, TX). Women recruited for the WM group were assigned to 200 mg supplemental Ca intake (WM NLCa). Each participant ingested 5 tablets/d (5 calcium tablets for the HiCa groups or 1 calcium and 4 placebo tablets for the NLCa groups). Pills were packaged in individual bags containing all 5 pills for a day. The participants were instructed to take 2 pills in the morning and 3 pills at night until completion of the study. The participants were counseled to consume 600 mg dietary Ca/d, and, if supplement compliance was 100%, total calcium intake (calcium supplement+MVi+dietary calcium intake) would be equivalent to 1800 mg/d and 1000 mg/d in the HiCa and NLCa groups, respectively. Compliance was assessed by monthly pill count, whereas dietary calcium intake was estimated from food records. A registered dietitian provided weekly weight loss counseling to the WL groups. Moderate energy restriction of 1200–1500 kcal/d with the use of American Diabetic Association exchange list and behavior modification was used to achieve weight loss or maintenance during the 6-mo study period. Weight was measured weekly for the first 2 mo and every other week thereafter. Women who did not lose enough weight (>1.5 kg) after 7 wk of counseling were excluded from the WL group. Women on weight maintenance were counseled to maintain weight and were weighed at monthly meetings. All groups were counseled to keep weekly 3-d food records to enhance weight loss counseling and assess dietary compliance. Measurements of bone density and hormones were taken before caloric restriction and after 6 mo of weight loss. In a subset of the participants, a calcium absorption test was performed and hormones were measured before and ≈6 wk after caloric restriction at the same time of their menstrual cycle and at a time when we anticipated greatest adherence to caloric restriction. In addition, serial measurements of bone turnover markers at baseline and during the intervention (weeks 1, 2, 4, 6, 10, 16, and 25) were collected.
Laboratory methods
Weight and height (measured with a balance beam scale and stadiometer, respectively; Detecto, Webb City, MO) were measured to the nearest 0.25 kg and cm, respectively. Dual-energy X-ray absorptiometry (DXA) (Lunar Prodigy Advanced; GE-Lunar, Madison, WI; CV: ≤ 1% for all sites) scans were performed by using enCORE 2004 software (version 8.10.027; GE-Lunar) before and 6 mo after caloric restriction or weight maintenance. Bone mineral content (BMC) and bone mineral density (BMD) at the femoral neck (FN), trochanter, total hip, ultradistal radius, 33% radius, lumbar spine (LS), and total body, as well as total body fat and lean mass, were determined.
Bone turnover markers were assessed in fasting urine and serum samples. Pyridinoline (CV: <8%) and deoxypyridinoline (CV: <10%) were measured in urine by reverse-phase HPLC and fluorescence detection (21) and normalized for creatinine excretion. Urinary calcium and creatinine were measured (no. 587 for calcium and no. 555 for creatinine; Sigma, St Louis, MO; CVs: <3.2% and < 11%, respecively) in 24-h urine samples. Serum osteocalcin was measured by radioimmunoassay (RIA) (BTI, Stoughton, MA; CV: <9%). Serum N-telopeptide of type I collagen (sNTx) was measured by enzyme-linked immunosorbent assay (Osteomark; OSTEX International Inc, Seattle, WA; CV: <4.6%). Serum 25-hydroxyvitamin D [25(OH)D] was measured by 125I RIA (DiaSorin, Stillwater, MI; CV: <12.5%). Estradiol, estrone, and cortisol were also measured by 125I RIA (DSL, Webster, TX; CV: <9.4%). Intact parathyroid hormone (PTH) was determined by immunoradiometric assay (DSL; CV: <5.2%). In addition, 1,25-dihydroxyvitamin D [1,25(OH)2D] was measured by 125I RIA after preliminary extraction procedure and subsequent purification by using C18OH “Extra Clean” cartridges (DiaSorin, Stillwater, MI; CV: <11.3%) on the day of the calcium absorption test.
Dual-stable isotope methods were used to determine true fractional calcium absorption (TFCA) at both baseline and ≈1 mo later. On the day of the calcium absorption test, the women were admitted at 0700 after an overnight fast. After blood collection (10 mL), participants were asked to void and then served a standard breakfast (170 mg Ca) to be consumed in its entirety. This meal contained 43Ca that had been mixed in one-half cup milk (150 mg Ca) and allowed to equilibrate for ≥12 h (overnight) before the test. Immediately after breakfast, 42Ca was injected intravenously over ≈3 min. Complete urine collection was monitored in each participant for the following 24 h, and the ratio of each isotope to 44Ca was determined in oxalate-precipitated aliquots of the pooled 24-h urine by using high-resolution inductively coupled plasma–mass spectrometry (ICP-MS) to calculate TFCA, as described previously (20, 22). The ICP-MS instrument precision and accuracy for this method is <±1% and the day-to-day CV is 1.2%.
Dietary intakes were estimated by analyzing food records (3-d averages) at baseline and during the study period. Diets were analyzed by using NUTRITIONIST PRO (version 1.3.36; First DataBank Inc, San Bruno, CA).
Statistical analysis
Inclusion criteria for the WL groups (and exclusion criteria for the WM groups) were weight loss >1.5% and 2.5% of initial body weight after 6 wk and 6 mo, respectively. In comparisons of small and unequal samples, nonparametric tests may be more powerful in detecting population differences when the normality assumption is not satisfied. Therefore, we used nonparametric tests (Wilcoxon's Mann-Whitney U test) for comparisons of bone mass and calcium absorption variables between the 3 intervention groups. Analysis of covariance (ANCOVA) was used to control for baseline differences. Additionally, a separate intention-to-treat (ITT) analysis (n = 58) with and without the use of success or failure of group assignment as a covariate (ANCOVA), as well as a completers analysis (n = 49), was performed for changes in bone mass. Subsequent post-hoc analysis with Tukey's test was performed when Kruskal-Wallis test results were P < 0.05. Repeated-measures analysis of variance (ANOVA) was used to assess changes in pyridinoline and deoxypyridinoline cross-links, osteocalcin, and PTH between the groups over time. Pearson's product-moment correlations were used to examine the relation between percentage changes in weight and bone mass and markers of bone turnover and hormones. To predict how weight loss influences bone mass and calcium absorption, simple linear regressions were performed. Stepwise multiple regression was performed on bone mass measurements that changed significantly or as a trend from baseline to final measurement, with percentage change in BMD and BMC as the dependent variables; independent variables included percentage change in weight, PTH, 25(OH)D, estradiol, and cortisol, as well as bone area for evaluating BMC changes. Stepwise regression was also performed by regressing calcium absorption (TFCA) on the independent variables PTH, 1,25(OH)2D, 25(OH)D, and estradiol. Analyses were performed with SAS software (version 9.1.3; SAS Institute Inc, Cary, NC). P values < 0.05 were considered statistically significant. Data are presented as means ± SDs, unless otherwise noted.
RESULTS
Study participants
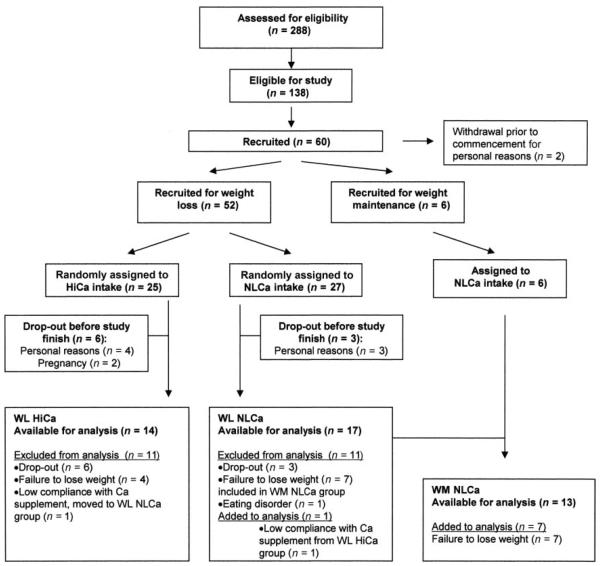
For the present study, 288 participants were screened, resulting in 58 women who were randomly assigned to either HiCa or NLCa intake (Figure 1). Nine women dropped out during the study because of personal reasons (n = 7) or pregnancy (n = 2). Four women assigned to the WL HiCa group who did not lose weight after the first 2 mo were excluded from the study. One woman was excluded from analysis due to disclosure of an eating disorder. Forty-four women completed the study. The women assigned to the WM group had to remain within 2.5% of their baseline body weight throughout the study period. Seven women originally assigned to the WL NLCa group who didn't lose >2.5% body weight within the first 2 mo met the criteria for the WM group and were allowed to continue in the study in this group assignment. This design, in addition to ITT and completers analysis, was important because we were interested in determining how weight loss affects bone, rather than who was successful at weight loss. These participants were analyzed as part of the WM group, and no baseline values were significantly different between the groups in the ITT analysis. In total, 31 women successfully completed the 6-mo weight loss program (24 white, 5 African American, 1 Hispanic, and 1 Asian), and 13 women (11 white, 1 African American, and 1 Asian) maintained their body weight. True FCA measurements were performed in 43 women at baseline and after 6 wk of caloric restriction. Nine samples were invalid due to use of different lots of stable isotopes at baseline and final measurements that were not compatible, and therefore TFCA analysis is reported in 34 women.
FIGURE 1.
Flow diagram of participants in study. Failure to lose weight was defined as weight loss of <2.5% of initial body weight. Low compliance was defined as those who took <60% of the calcium supplement. HiCa, high calcium; NLCa, normal calcium; WL, weight loss; WM, weight maintenance.
Baseline characteristics
The baseline characteristics of women (age: 38.0 ± 6.4 y, range 24–49 y; BMI 27.7 ± 2.1) included in the analysis are presented in Table 1 and Table 2. Significant differences (P < 0.05) in lumbar spine BMD and BMC and FN BMC were observed between the groups at baseline (Table 1). Due to a stabilization period to adjust to intakes of 1.0 g Ca/d and 1.8 g Ca/d before the beginning of weight loss, we observed significant (P < 0.02) differences in TFCA and estimated amounts of calcium absorbed between groups at baseline (Table 2). No other baseline characteristics differed significantly among the entire study group or the subset who participated in calcium absorption measurements. Participants were recruited every 6 mo, which resulted in different baseline variables depending on whether participants were recruited in the early fall or late winter months. Women who started weight loss in the early fall (n = 25) had significantly lower (P < 0.03) deoxypyridinoline concentrations (7.5 ± 2.2 nmol/mmol) than did the women who started in the late winter (n = 19; 9.4 ± 2.7 nmol/mmol). During dieting, the only variable that was significantly affected by the season of recruitment was serum 25(OH)D (P < 0.001). Values of 25(OH)D were 73.4 ± 22.2 nmol/L for groups beginning in late winter and increased by 19.8 ± 18.2% (P < 0.001) over 6 mo compared with 82.1 ± 20.5 nmol/L for groups beginning in the fall that showed a change of 1.1 ± 17.9%.
TABLE 1.
Bone and body-composition measurements at baseline and percentage changes after 6 mo of weight maintenance (WM) or weight loss (WL) at 2 calcium intakes1
| WL high calcium (n = 14) |
WL normal calcium (n = 17) |
WM normal calcium (n = 13) |
|||||
|---|---|---|---|---|---|---|---|
| Baseline | Percentage change | Baseline | Percentage change | Baseline | Percentage change | P 2 | |
| Body weight (kg) | 73.2 ± 4.4 | −8.1 ± 4.03,4 | 71.4 ± 6.7 | −6.6 ± 2.43,4 | 73.4 ± 5.0 | −0.1 ± 2.3 | < 0.0001 |
| Lean mass (kg) | 42.0 ± 3.6 | −2.0 ± 2.43 | 40.8 ± 4.4 | −4.4 ± 4.33,4 | 42.5 ± 3.4 | 0.4 ± 4.2 | 0.015 |
| Fat mass (kg) | 28.4 ± 2.4 | −17.9 ± 10.23,4 | 28.1 ± 4.2 | −11.4 ± 6.03,4 | 28.2 ± 4.8 | −0.6 ± 4.9 | < 0.0001 |
| BMD (g/cm2) | |||||||
| Femoral neck | 1.02 ± 0.13 | 1.5 ± 1.8 | 0.99 ± 0.11 | −0.5 ± 3.3 | 0.97 ± 0.14 | 1.0 ± 4.0 | 0.087 |
| Trochanter | 0.85 ± 0.10 | 1.2 ± 2.2 | 0.80 ± 0.01 | 0.2 ± 2.6 | 0.81 ± 0.12 | 1.5 ± 4.1 | 0.660 |
| Total hip | 1.07 ± 0.12 | 0.8 ± 1.4 | 1.03 ± 0.09 | 1.2 ± 0.9 | 1.03 ± 0.14 | 0.3 ± 1.9 | 0.106 |
| UD radius | 0.42 ± 0.04 | −1.5 ± 13.4 | 0.39 ± 0.06 | 11.6 ± 16.1 | 0.39 ± 0.06 | 1.1 ± 17.5 | 0.080 |
| 33% radius | 0.71 ± 0.06 | 2.2 ± 3.33,5 | 0.70 ± 0.06 | −1.4 ± 3.4 | 0.70 ± 0.05 | 0.6 ± 3.8 | 0.047 |
| Lumbar spine6 | 1.33 ± 0.19 | −0.1 ± 2.9 | 1.20 ± 0.13 | −1.0 ± 4.3 | 1.20 ± 0.12 | −0.2 ± 3.9 | 0.831 |
| Total body | 1.21 ± 0.06 | 0.6 ± 0.73 | 1.17 ± 0.08 | −0.2 ± 1.5 | 1.20 ± 0.07 | −1.1 ± 3.3 | 0.028 |
| BMC (g) | |||||||
| Femoral neck6 | 4.94 ± 0.65 | 1.6 ± 5.5 | 4.35 ± 0.43 | 5.8 ± 9.5 | 4.51 ± 0.65 | 3.8 ± 7.6 | 0.314 |
| Trochanter | 9.23 ± 1.43 | 3.3 ± 6.7 | 8.79 ± 1.37 | −2.2 ± 6.3 | 8.92 ± 1.99 | 5.0 ± 6.93,5 | 0.015 |
| Total hip | 31.8 ± 4.0 | 1.5 ± 2.0 | 29.7 ± 2.5 | 1.1 ± 2.1 | 29.9 ± 4.1 | 0.8 ± 3.1 | 0.721 |
| UD radius | 1.34 ± 0.16 | −1.5 ± 13.5 | 1.30 ± 0.16 | −5.4 ± 9.3 | 1.32 ± 0.16 | −3.3 ± 6.1 | 0.586 |
| 33% radius | 1.86 ± 0.23 | −4.5 ± 4.23,5 | 1.71 ± 0.21 | 4.8 ± 8.93 | 1.81 ± 0.25 | −1.8 ± 8.8 | 0.005 |
| Lumbar spine6 | 56.0 ± 9.0 | 0.8 ± 3.6 | 49.4 ± 8.1 | 0.4 ± 3.6 | 49.4 ± 6.2 | 1.2 ± 6.0 | 0.978 |
| Total body | 2717 ± 297 | −1.3 ± 2.4 | 2513 ± 281 | 0.0 ± 2.4 | 2565 ± 232 | −0.1 ± 2.4 | 0.272 |
Normal calcium intake was 1.0 g/d; high calcium intake was 1.8 g/d. UD, ultradistal radius.
Comparison of percentage change between the 3 groups by nonparametric one-factor ANOVA.
Significantly different from baseline, P < 0.05.
Change was significantly different from WM group, P < 0.05.
Change was significantly different from WL normal-calcium group, P < 0.05.
There was a significant difference between the groups at baseline, P < 0.05.
TABLE 2.
Baseline values and percentage changes after 6 wk and 6 mo due to weight loss (WL) or weight maintenance (WM) in premenopausal women at 2 calcium intakes1
| WL high calcium (n = 14) |
WL normal calcium (n = 17) |
WM normal calcium (n = 13) |
|||||
|---|---|---|---|---|---|---|---|
| Baseline | Percentage change | Baseline | Percentage change | Baseline | Percentage change | P 2 | |
| Calcium absorption, vitamin D, and PTH after 6 wk3 | |||||||
| TFCA (%)4 | 27.1 ± 6.2a,b | −30.6 ± 22.05,6 | 27.0 ± 5.7a | −11.6 ±22.6 | 33.6 ± 5.6b | −9.2 ± 13.3 | 0.049 |
| Estimated | |||||||
| Calcium absorbed (mg/d)4 | 492 ± 178a | −30.3 ± 32.5 | 282 ± 171b | −3.0 ± 35.7 | 371 ± 103a,b | −17.3 ± 28.4 | 0.077 |
| 1,25(OH)2D (pmol/L) | 118.3 ± 19.5 | −2.0 ± 32.3 | 110.0 ± 41.1 | 2.3 ± 38.2 | 112.3 ± 43.7 | 11.0 ± 62.0 | 0.726 |
| 25(OH)D (nmol/L) | 72.4 ± 16.0 | 27.9 ± 33.45 | 74.9 ± 27.2 | 26.6 ± 40.15 | 92.4 ± 35.4 | −0.5 ± 29.7 | 0.058 |
| PTH (pmol/L) | 3.7 ± 1.9 | 4.1 ± 40.2 | 3.6 ± 1.6 | 4.6 ± 28.0 | 3.3 ± 1.7 | 5.8 ± 57.6 | 0.743 |
| Hormones and bone markers after 6 mo | |||||||
| 25(OH)D (nmol/L) | 75.1 ± 22.5 | 9.1 ± 23.1 | 81.1 ± 17.2 | 10.6 ± 22.5 | 74.4 ± 27.0 | 8.0 ± 13.6 | 0.964 |
| PTH (pmol/L) | 3.5 ± 1.4 | 21.8 ± 41.1 | 3.4 ± 1.4 | −3.1 ± 40.0 | 4.2 ± 2.0 | −3.6 ± 25.5 | 0.238 |
| Estradiol (pmol/L) | 131.8 ± 69.4 | 41.4 ± 58.2 | 196.4 ± 140.6 | 59.9 ± 158.2 | 194.2 ± 115.3 | 78.7 ± 226.0 | 0.569 |
| Cortisol (nmol/L) | 295.2 ± 85.5 | 22.6 ± 39.0 | 306.2 ± 104.8 | 16.4 ± 54.1 | 322.8 ± 113.1 | 14.8 ± 43.4 | 0.730 |
| Pyridinium:creatinine (nmol/mmol) | 26.7 ± 7.5 | 9.6 ± 26.0 | 31.1 ±8.4 | −2.7 ± 26.4 | 27.8 ± 6.6 | 7.9 ± 29.2 | 0.329 |
| Deoxypyridinoline: creatinine (nmol/mmol) | 7.8 ± 1.9 | 6.6 ± 29.8 | 8.5 ± 2.6 | 9.3 ± 33.6 | 8.6 ± 3.3 | −3.7 ± 17.2 | 0.988 |
| sNTx (nmol BCE) | 13.5 ±3.3 | 3.6 ± 20.0 | 14.6 ± 4.3 | 3.3 ± 34.4 | 13.7 ± 3.6 | 2.4 ± 22.6 | 0.985 |
| Osteocalcin (nmol/L) | 1.3 ± 0.2 | −7.5 ± 14.6 | 1.3 ± 0.2 | 6.2 ± 12.7 | 1.3 ± 0.3 | 6.5 ± 14.6 | 0.172 |
Normal calcium intake was 1.0 g/d; high calcium intake was 1.8 g/d. PTH, parathyroid hormone; TFCA, true fractional calcium absorption; 25(OH)D, 25-hydroxyvitamin D; 1,25(OH2)D, 1,25-dihydroxyvitamin D; sNTx, serum JV-telopeptide; BCE, bone collagen equivalents.
Comparison of percentage changes between the 3 groups (nonparametric one-factor ANOVA).
For a subset: n = 9 in the WL high-calcium group; n = 15 in the WL normal-calcium group; and n = 10 in the WM normal-calcium group.
There was a significant difference between groups at baseline, P < 0.05. Values with different superscript letters are significantly different, P < 0.05.
Significantly different from baseline value, P < 0.05.
Significantly different from WM group, P < 0.05.
Weight and body-composition changes
Baseline body weight and changes after 6 mo are presented in Table 1. Women in the WL groups lost 7.2 ± 3.3% body weight (5.5 ± 3.2 kg) after 6 mo. No significant difference in weight loss was observed between those consuming high calcium (−5.9 ± 3.0 kg) and those consuming normal calcium (−4.7 ± 1.8 kg) at 6 mo, but there was an interaction effect of calcium and time on weight loss (P < 0.01) such that the high calcium group lost more weight over the measured intervals. Women in the WM group maintained weight (−0.1 ± 1.7 kg) during the study period. As expected, weight loss was largely due to a decrease in body fat mass (−14.3 ± 8.7%; P < 0.0001) and a small decrease in lean body mass (−3.3 ± 3.7%; P < 0.001).
Bone
Changes in BMD and BMC are shown in Table 1. Significant differences (P < 0.05) in BMD changes at the 33% radius and total body were observed between the groups, and a trend toward a difference was observed at the FN and ultradistal radius (Table 1). Furthermore, BMC changes differed (P < 0.02) between all groups at the trochanter and one-third radius.
Women who had lost weight and consumed high calcium increased BMD (P < 0.05) at the one-third radius and total body compared with baseline (Table 1). In addition, the FN BMD increased in the high-calcium group (P < 0.05) compared with baseline (not shown), although the overall P value was a trend. The WM group showed no significant changes in BMD, but increased trochanter BMC after 6 mo.
ITT and completers analysis, which included women who did not lose weight in the WL groups, showed that the WL groups differed from the WM group (P < 0.05) for changes in total hip BMD and BMC and total body BMD (data not shown).
Nutrient intake
Nutrient intakes are shown in Table 3. Dietary intake of calcium during the study period was 730 ± 268 mg/d and supplement compliance was 93 ± 12%. Total calcium intake during the study period was ≈1.1 and 1.8 g/d in the NL and HiCa groups, respectively. No significant differences in other nutrient intakes were observed between the groups at baseline. Compared with prestabilization calcium intake, there was a 125 ± 90% increase in calcium intake (P < 0.001) in the HiCa group (Table 3). This increase in calcium intake in the HiCa group was significantly greater (P < 0.0001) than the NLCa groups that showed no significant change during stabilization. The WL groups showed reduced (P < 0.05) intake of calories and fat compared with baseline.
TABLE 3.
Nutrient intakes at baseline and percentage changes after 6 mo of weight maintenance (WM) or weight loss (WL) in premenopausal women at 2 calcium intakes1
| WL high calcium (n = 14) |
WL normal calcium (n = 17) |
WM normal calcium (n = 13) |
|||||
|---|---|---|---|---|---|---|---|
| Baseline | Percentage change | Baseline | Percentage change | Baseline | Percentage change | P 2 | |
| Energy (kcal) | 1707 ± 4613 | −19.4 ± 31.94 | 1885 ± 587 | −25.4 ± 20.14,5 | 1708 ± 461 | −4.9 ± 11.9 | 0.029 |
| Protein (g) | 66.4 ± 18.0 | −1.4 ± 30.5 | 76.2 ± 29.1 | −12.9 ± 31.5 | 76.9 ± 21.9 | −7.9 ± 24.7 | 0.501 |
| Fat (g) | 64.5 ± 26.1 | −25.9 ± 32.04 | 72.9 ± 25.3 | −38.1 ± 23.04,5 | 66.2 ± 34.5 | −6.7 ± 15.1 | 0.007 |
| Carbohydrates (g) | 212.6 ± 65.7 | −14.1 ± 35.6 | 231.0 ± 80.9 | −18.7 ± 23.6 | 205.2 ± 42.4 | −6.5 ± 21.1 | 0.304 |
| Calcium (mg)6 | 1821 ± 366 | 4.0 ± 14.7 | 1088 ± 489 | 6.8 ± 35.6 | 1156 ± 397 | 0.4 ± 18.2 | 0.799 |
| Phosphorus (mg) | 866 ± 270 | 0.1 ± 26.7 | 1037 ± 502 | −8.0 ± 37.5 | 1036 ± 427 | 0.8 ± 23.2 | 0.669 |
| Vitamin D (μg) | 1.6 ± 1.1 | 5.7 ± 12.7 | 2.1 ± 3.0 | −0.2 ± 16.4 | 2.4 ± 1.7 | −0.9 ± 8.2 | 0.357 |
| Magnesium (mg) | 163.8 ± 55.8 | 11.9 ±30.3 | 198.5 ± 82.9 | −3.4 ± 20.9 | 244.9 ± 163.7 | −1.7 ±22.3 | 0.197 |
| Sodium (mg) | 2408 ± 828 | −2.0 ± 33.1 | 2986 ± 1338 | −5.2 ± 67.5 | 2487 ± 826 | −8.4 ± 18.8 | 0.467 |
| Vitamin K (μg) | 38.8 ± 40.9 | 8.4 ± 65.2 | 51.0 ± 45.0 | 10.5 ± 68.7 | 51.9 ± 33.3 | 54.1 ± 161.1 | 0.436 |
Nutrient intake was estimated from 3-d food records at baseline and from two 3-d food records at 6 mo. Normal calcium intake was 1.0 g/d; high calcium intake was 1.8 g/d. Includes 48 mg phosphorus, 10 μg vitamin D, 100 mg magnesium, or 10 μg vitamin K from multivitamin-minerals; does not include salt from shaker. No significant differences were observed between the groups at baseline.
Comparison of percentage changes between the 3 groups (nonparametric one-factor ANOVA).
(all such values).
Significantly different from baseline value, P < 0.05.
Significantly different from WM, P < 0.05.
Includes supplemental calcium at baseline and during study period of 0.4 g/d (normal calcium) and 1.2 g/d (high calcium). Mean (±SD) calcium intakes before supplementation (prestabilization): WL high calcium: 951 ± 334 mg/d; WL normal calcium; 1155 ± 500 mg/d; and WM normal calcium; 1024 ± 381 mg/d.
Hormones, bone turnover, calcium absorption, and urinary excretion
Changes in calcium absorption, hormones, and bone turnover are shown in Table 2. Serum 25(OH)D concentrations increased due to weight loss compared with baseline after 6 wk (P < 0.05), and these changes tended to differ from the WM group (P < 0.06). In contrast, none of the groups showed a significant change in serum 25(OH)D concentrations after 6 mo (Table 2). No significant differences in any hormonal responses were observed between the groups throughout the study period. Serial measures of osteocalcin tended (P < 0.08) to decrease in the WL groups and did not significantly change in the WM group after 6 mo. Also, serial markers of bone resorption, serum NTx, urinary pyridinoline and deoxypyridinoline, bone formation, and osteocalcin did not significantly differ between the groups or compared with baseline.
After 6 wk of caloric restriction, TFCA decreased (P < 0.05) during caloric restriction in the women who consumed high calcium during weight loss compared with those who consumed normal calcium during weight loss and compared with those on weight maintenance. The estimated amounts of calcium absorbed tended (P < 0.06) to be greater in the HiCa group (309.8 ± 91.4 mg/d) than in the WL NLCa group (238.4 ± 81.3 mg/d), but not compared with the WM group (287.3 ± 61.1 mg/d).
Women were stabilized at calcium intakes of 1.0 g/d or 1.8 g/d ≈4 wk before the first 24-h urine collection and averaged 195.6 ± 97.6 mg Ca/d for all women at baseline. As a result of this calcium stabilization before baseline, urinary calcium excretion was not expected to and did not increase in any of the groups at 6 mo. Furthermore, during normal intake of calcium, urinary calcium excretion did not differ significantly in women losing or maintaining weight. Creatinine excretion (24-h collection) was 1122 ± 283 mg/d at baseline and did not change significantly (1.2 ± 34.5%) with weight loss, which is consistent with the small decrease in lean mass.
Correlations: simple linear and stepwise multiple regression
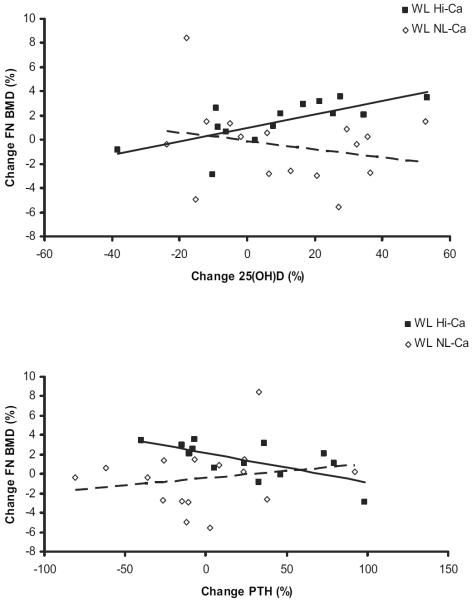
We analyzed changes in weight loss, body composition, markers of bone turnover, and hormones within each group. Most correlations were found for the WL HiCa group, because this group experienced the greatest changes in measurements. Weight loss tended to be associated with a decrease in osteocalcin (r = 0.339, P < 0.07). The increase in 25(OH)D correlated significantly (P < 0.005) with an increase in FN BMD (r = 0.705) and a decrease in sNTx (r=−0.728) in the HiCa group, but not in the WL group with normal calcium intake (r=−0.232, P = 0.386) (slopes tended to differ for FN BMD, P < 0.06; Figure 2A). Furthermore, the change in serum PTH was negatively associated with the changes in FN BMD during weight loss and high calcium intake (r=−0.681, P < 0.01, Figure 2B), but not with NL calcium intake.
FIGURE 2.
Relation between changes in serum 25-hydroxyvitamin D [25(OH)D] or parathyroid hormone (PTH) and femoral neck bone mineral density (FN BMD) in the weight loss high calcium (WL HiCa) and the WL normal calcium (NLCa) groups. The relation between 25(OH)D and FN BMD was significant (r=0.705, P<0.005) for the WL HiCa group, but not for the WL NLCa group (r = −0.232, P = NS). A trend (P < 0.06) for a difference in the slopes between the groups was observed. Changes in PTH and FN BMD were observed only in the WL Hi-Ca group (WL HiCa: r = −0.681, P < 0.01; and WL NLCa: r = 0.187, P = NS) without significant differences between the slopes after 6 mo of weight loss. ■ and solid line, WL HiCa group (n = 14), ◇ and dashed line, WL NLCa group (n = 17). Stepwise regression analysis was done with percentage change in BMD as the dependent variable, and independent variables included percentage change in weight, PTH, 25(OH)D, estradiol, and cortisol.
Significant results for stepwise multiple regression analysis were found in the HiCa group. An increase in 25(OH)D (partial R2 = 0.498, P < 0.005) and decrease in PTH (partial R2 = 0.189, P < 0.05) together explained 68.6% of the variance in FN BMD. Stepwise multiple regression analysis for TFCA across all 3 groups showed that 36% of the variance in the TFCA changes was explained by calcium intake (r = 0.216, P < 0.01) and estradiol changes (r = 0.145, P < 0.02).
DISCUSSION
The present study showed that there is no bone loss with moderate weight reduction in healthy overweight premenopausal women at either 1 g Ca/d or 1.8 g Ca/d intake. These findings are important because there are numerous studies showing bone loss with weight reduction, but the population is typically older and/or weight loss greater and more rapid. Few studies have investigated the effects of weight loss on bone in premenopausal women, and these studies show contradicting results (1–3, 5). Our goal in this 6-mo weight-loss trial was to specifically address the overweight population because they have more recently been defined as a population that should lose weight (14) and who may be at greater risk of bone loss than are obese persons due to weight reduction (14). Another goal was to examine calcium intake at recommended or high levels and its role in preventing bone loss due to weight reduction (1, 5, 7, 19). In our recent publication (12), we found that 1 g Ca/d was not adequate to prevent bone loss in overweight postmenopausal women during weight loss. In the current study, intake of 1 g Ca/d was adequate to prevent bone loss, and women who consumed 1.8 g Ca/d showed a small increase in BMD at several sites.
In previous controlled weight loss trials in postmenopausal women or mixed populations, we and others consistently find bone loss due to weight reduction (11–13). However, results of bone loss in premenopausal women are controversial. For example, in a previous controlled weight loss trial conducted in obese premenopausal women, we found no decrease in BMD and BMC with or without calcium supplementation (5). However, in a 1.5 y study of lean and overweight (BMI ≈25) women beginning at 47 y of age, a 5% weight loss resulted in a small but significant loss of BMD at the hip (0.8% loss), but not the spine, compared with weight-stable women (4). Weight loss trials without a control group and calcium supplementation conducted in premenopausal women report different results. For example, Ramsdale et al (1) found that a 5% weight loss reduced calcium intake to 866 mg/d and resulted in a slight decrease in BMD at the total body (−0.7%) and lumbar spine (−0.5%) in women (aged 18–44 y). A 3-mo very-low-energy diet and 910 mg Ca/d conducted by Fogelholm et al (2) resulted in loss of BMD and BMC at various sites (1–6%). Hence, a rapid 14.3% weight loss (≈5%/mo) (2) compared with slower weight loss in the current trial (≈1.2%/mo) may be responsible for the bone loss. Another short-term (15 wk) weight-loss trial conducted by Van Loan et al (3) reported a decrease in total body BMD of 1.7% without corresponding BMC changes in 14 women after ≈10% weight loss. Calcium intake was not reported in this study (3), and the authors suggest that the observed decrease in BMD in these women may be due to measurement artifact. Because a complete bone remodeling cycle, including activation, resorption, and formation, takes ≈6 mo in older individuals (23), it is possible that the observed small changes in bone mass with the HiCa diet in the present study are temporary due to the bone remodeling transient. Whether the changes are, in fact, significant during weight loss should be addressed in a longer-term study. Nevertheless, others have examined weight-stable young women over 5 y and found that modest increases in calcium intake can positively affect bone (24).
Calcium absorption has previously been shown to decrease with weight loss (20, 25), and under conditions of adequate vitamin D status we have found that estrogen is an important predictor of calcium absorption. In the present study, an important goal was to evaluate the effects of caloric restriction on calcium absorption in estrogen-replete women. Calcium absorption decreased in all 3 groups over the 6-wk period, regardless of weight change. We showed a greater decrease in TFCA in the high than in the normal calcium group. This was unexpected, because women were stabilized to high calcium intakes 3–4 wk before their baseline measurement, which should have been sufficient time to adapt to a new level of calcium intake (26). Heaney (27) reported TFCA from observational studies in premenopausal women to be ≈23% at 1 g Ca/d and ≈18% at higher calcium intakes (≈1.8 g Ca/d). Based on these data, one can estimate that increasing calcium intake from 1.0 g/d to 1.8 g/d results in a decrease in TFCA by about 22%, which is comparable to the 30% decrease in TFCA observed in the present study. The amount of total absorbed calcium is a key factor in attaining calcium balance and thereby in the prevention of bone loss. In the present study, it was estimated that the WL groups absorbed 238–310 mg Ca/d after 6 wk of weight loss. In women 35–50 y of age (28), calcium balance may be attained if absorbed calcium is above ≈230 mg/d. We did not measure calcium balance in the present study, but considering the amounts of total calcium absorbed (all >238 mg/d), women in all 3 groups in this study appear to have been in calcium balance, which is consistent with our findings of no bone loss.
Another important goal in the current study was to determine whether normal or high calcium intake would be sufficient to suppress bone turnover during weight loss. Previous weight-loss studies showed a greater increase in bone resorption than in formation with intakes of 0.6–0.8 g Ca/d (5, 7, 13, 29, 30), but not with higher calcium intakes (12, 30). The absence of any rise in bone turnover is consistent with the higher calcium intake in the present study (1.1–1.8 g/d) than those of previous trials. In the present study, we observed a decrease in the bone formation marker, osteocalcin, with weight loss compared with weight-stable women. Chronic food restriction has been shown to decrease circulating osteocalcin concentrations in clinical weight-loss trials (13, 30). It is possible that the uncoupling of bone formation from resorption with longer term weight-loss trials could ultimately result in bone loss.
Hormones regulating bone changes have not been examined in previous weight-loss trials in premenopausal women and were a focus in the present study. We observed an increase in serum vitamin D concentrations with weight loss after 6 wk, but not after 6 mo. Others have found a rise in 25(OH)D with loss of adipose tissue in rats (31, 32). The WL group that consumed high calcium was the only group to show a strong correlation between increased serum 25(OH)D and increased FN-BMD. It is possible that a high calcium intake combined with an increase in vitamin D concentrations is beneficial to bone mass. Two potential mechanisms may explain this finding. Higher serum concentrations of vitamin D in the HiCa group were associated with a lower rate of bone resorption and tended to decrease urinary calcium excretion. Calcium may function in an enabling mode, permitting the skeleton to respond to hormonal cues (33), which may have contributed to the bone response in the HiCa group. Nevertheless, the relation between 25(OH)D and FN-BMD could also be an artifact due to the small sample size. We hypothesized that calcium requirements would be higher than those recommended during weight loss, yet both an absence of bone loss and a higher PTH and bone turnover suggest that our original hypothesis was not true. In addition, even though we did not design the study to examine the effect of calcium on body weight and fat and did not find a significant weight difference between the HiCa and NLCa groups after 6 mo, we did find that repeated measurements over time showed the HiCa group lost more body weight than did the NLCa group. This differs from our previous findings (12, 34), and the literature examining calcium during weight loss remains controversial on this subject (35, 36).
There are some limitations of the present study. There are concerns about the validity of DXA measurements in heavier people and in weight-loss studies because of size-related artifacts (37–39). We suggest that measurement artifacts due to weight were minimal in the current study, due to a lower baseline body weight, no extremes in fat distribution because the BMI of the women was within a narrow range (25 to 29.9), small tissue changes with moderate weight loss (ie, of ≈7%), and most importantly the inclusion of a weight-loss control group (10, 40–42). Furthermore, we reported both BMD and BMC changes in Table 1 to improve interpretation of bone changes. Nevertheless, the DXA fan beam may overestimate true BMD changes due to beam hardening (32). Also, because of higher variability of bone changes at the radius than at other sites, despite the use of the same machine and technician, we suggest that interpretation is limited for this peripheral site. This is possibly due to the difficulty in obtaining a standardized region of interest, less reliable data for percentage change due to its low BMD and mass, or variable trabecular content (43, 44). In one study, the diagnostic validity proved to be substantially better at a highly trabecular region of the distal radius (45).
In conclusion, we showed that dieting overweight premenopausal women do not lose bone with intakes of 1.0 or 1.8 g Ca/d, which may be explained by sufficient amounts of absorbed calcium. Furthermore, bone turnover was not up-regulated, and the calcium-PTH axis was not significantly increased because of weight loss. We suggest that overweight premenopausal women do not lose bone with moderate weight loss when consuming the recommended intake for calcium.
Acknowledgments
We thank G Regis-Andrews, B Dobrzynski, and A Charles for their excellent clinical care and the invaluable efforts of the volunteers in this study.
Footnotes
From Rutgers University, New Brunswick, NJ (CSR, NvT, HAS, MPF, RMS, and SAS), the New York Medical College, Valhalla, NY (YS), and the University of Medicine and Dentistry, New Brunswick, NJ (TS)
Supported by NIH grant AG-12161 (to SAS).
Address reprint requests SA Shapses, Department of Nutritional Sciences, Rutgers University, 96 Lipman Drive, New Brunswick, NJ 08901-8525. shapses@aesop.rutgers.edu.
SAS designed the study and contributed to the lab and data analysis and writing the manuscript. NvT contributed to the implementation of the study, counseling of the volunteers, record keeping, and data collection. CSR contributed to the implementation of the study, counseling of the volunteers, record keeping, and data collection and also performed data management, laboratory and statistical analysis, and was responsible for cowriting the manuscript. YS contributed to the study design, statistical analysis and data management. HA-S helped to implement the study protocol and was responsible for the laboratory analysis, data entry, and quality control. RMS and MPF were also responsible for the laboratory analysis, data entry, and quality control. TS analyzed bone density scans and contributed to data interpretation and research for writing the discussion. Neither the authors nor those acknowledged for their efforts in the study had any potential conflicts of interest.
REFERENCES
- 1.Ramsdale SJ, Bassey EJ. Changes in bone mineral density associated with dietary-induced loss of body mass in young women. Clin. Sci (Lond) 1994;87:343–8. doi: 10.1042/cs0870343. [DOI] [PubMed] [Google Scholar]
- 2.Fogelholm GM, Sievanen HT, Kukkonen-Harjula TK, Pasanen ME. Bone mineral density during reduction, maintenance and regain of body weight in premenopausal, obese women. Osteoporos Int. 2001;12:199–206. doi: 10.1007/s001980170130. [DOI] [PubMed] [Google Scholar]
- 3.Van Loan MD, Johnson HL, Barbieri TF. Effect of weight loss on bone mineral content and bone mineral density in obese women. Am J Clin Nutr. 1998;67:734–8. doi: 10.1093/ajcn/67.4.734. [DOI] [PubMed] [Google Scholar]
- 4.Salamone LM, Cauley JA, Black DM, et al. Effect of a lifestyle intervention on bone mineral density in premenopausal women: a randomized trial. Am J Clin Nutr. 1999;70:97–103. doi: 10.1093/ajcn/70.1.97. [DOI] [PubMed] [Google Scholar]
- 5.Shapses SA, Von Thun NL, Heymsfield SB, et al. Bone turnover and density in obese premenopausal women during moderate weight loss and calcium supplementation. J Bone Miner Res. 2001;16:1329–36. doi: 10.1359/jbmr.2001.16.7.1329. [DOI] [PubMed] [Google Scholar]
- 6.Meyer HE, Tverdal A, Selmer R. Weight variability, weight change and the incidence of hip fracture: a prospective study of 39,000 middle-aged Norwegians. Osteoporos Int. 1998;8:373–8. doi: 10.1007/s001980050077. [DOI] [PubMed] [Google Scholar]
- 7.Ricci TA, Heymsfield SB, Pierson RN, Jr, Stahl T, Chowdhury HA, Shapses SA. Moderate energy restriction increases bone resorption in obese postmenopausal women. Am J Clin Nutr. 2001;73:347–52. doi: 10.1093/ajcn/73.2.347. [DOI] [PubMed] [Google Scholar]
- 8.Chao D, Espeland MA, Farmer D, et al. Effect of voluntary weight loss on bone mineral density in older overweight women. J Am Geriatr Soc. 2000;48:753–9. doi: 10.1111/j.1532-5415.2000.tb04749.x. [DOI] [PubMed] [Google Scholar]
- 9.Compston JE, Laskey MA, Croucher PI, Coxon A, Kreitzman S. Effect of diet-induced weight loss on total body bone mass. Clin. Sci (Lond) 1992;82:429–32. doi: 10.1042/cs0820429. [DOI] [PubMed] [Google Scholar]
- 10.Jensen LB, Quaade F, Sorensen OH. Bone loss accompanying voluntary weight loss in obese humans. J Bone Miner Res. 1994;9:459–63. doi: 10.1002/jbmr.5650090404. [DOI] [PubMed] [Google Scholar]
- 11.Jensen LB, Kollerup G, Quaade F, Sorensen OH. Bone minerals changes in obese women during a moderate weight loss with and without calcium supplementation. J Bone Miner Res. 2001;16:141–7. doi: 10.1359/jbmr.2001.16.1.141. [DOI] [PubMed] [Google Scholar]
- 12.Riedt CS, Cifuentes M, Stahl T, Chowdhury HA, Schlussel Y, Shapses SA. Overweight postmenopausal women lose bone with moderate weight reduction and 1 g/day calcium intake. J Bone Miner Res. 2005;20:455–63. doi: 10.1359/JBMR.041132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ricci TA, Chowdhury HA, Heymsfield SB, Stahl T, Pierson RN, Jr, Shapses SA. Calcium supplementation suppresses bone turnover during weight reduction in postmenopausal women. J Bone Miner Res. 1998;13:1045–50. doi: 10.1359/jbmr.1998.13.6.1045. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen TV, Sambrook PN, Eisman JA. Bone loss, physical activity, and weight change in elderly women: the Dubbo Osteoporosis Epidemiology Study. J Bone Miner Res. 1998;13:1458–67. doi: 10.1359/jbmr.1998.13.9.1458. [DOI] [PubMed] [Google Scholar]
- 15.Edelstein SL, Barrett-Connor E. Relation between body size and bone mineral density in elderly men and women. Am J Epidemiol. 1993;138:160–9. doi: 10.1093/oxfordjournals.aje.a116842. [DOI] [PubMed] [Google Scholar]
- 16.Harris SS, Dawson-Hughes B. Weight, body composition, and bone density in postmenopausal women. Calcif Tissue Int. 1996;59:428–32. doi: 10.1007/BF00369205. [DOI] [PubMed] [Google Scholar]
- 17.Wardlaw GM. Putting body weight and osteoporosis into perspective. Am J Clin Nutr. 1996;63(suppl):433S–6S. doi: 10.1093/ajcn/63.3.433. [DOI] [PubMed] [Google Scholar]
- 18.Feher T, Bodrogi L, Vallent K, Ribai Z. Role of human adipose tissue in the production and metabolism of steroid hormones. Endokrinologie. 1982;80:173–80. in German. [PubMed] [Google Scholar]
- 19.Teegarden D. Calcium intake and reduction in weight or fat mass. J Nutr. 2003;133(suppl):249S–51S. doi: 10.1093/jn/133.1.249S. [DOI] [PubMed] [Google Scholar]
- 20.Cifuentes M, Riedt CS, Brolin RE, Field MP, Sherrell RM, Shapses SA. Weight loss and calcium intake influence calcium absorption in over-weight postmenopausal women. Am J Clin Nutr. 2004;80:123–30. doi: 10.1093/ajcn/80.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eyre DR, Koob TJ, Van Ness KP. Quantitation of hydroxypyridinium crosslinks in collagen by high-performance liquid chromatography. Anal Biochem. 1984;137:380–8. doi: 10.1016/0003-2697(84)90101-5. [DOI] [PubMed] [Google Scholar]
- 22.Field MP, Cifuentes M, Sherell RM, Shapses SA. Determination of Ca isotope ratios in metabolic studies using sector field HR-ICPMS. J Anal Atomic Spec. 2003;18:727–33. [Google Scholar]
- 23.Heaney RP. The bone remodeling transient: interpreting interventions involving bone-related nutrients. Nutr Rev. 2001;59:327–34. doi: 10.1111/j.1753-4887.2001.tb06957.x. [DOI] [PubMed] [Google Scholar]
- 24.Recker RR, Davies KM, Hinders SM, Heaney RP, Stegman MR, Kimmel DB. Bone gain in young adult women 1. JAMA. 1992;268:2403–8. [PubMed] [Google Scholar]
- 25.Cifuentes M, Morano AB, Chowdhury HA, Shapses SA. Energy restriction reduces fractional calcium absorption in mature obese and lean rats. J Nutr. 2002;132:2660–6. doi: 10.1093/jn/132.9.2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dawson-Hughes B, Harris S, Kramich C, Dallal G, Rasmussen HM. Calcium retention and hormone levels in black and white women on high- and low-calcium diets. J Bone Miner Res. 1993;8:779–87. doi: 10.1002/jbmr.5650080702. [DOI] [PubMed] [Google Scholar]
- 27.Heaney RP, Recker RR, Stegman MR, Moy AJ. Calcium absorption in women: relationships to calcium intake, estrogen status, and age. J Bone Miner Res. 1989;4:469–75. doi: 10.1002/jbmr.5650040404. [DOI] [PubMed] [Google Scholar]
- 28.Heaney RP, Recker RR, Saville PD. Calcium balance and calcium requirements in middle-aged women. Am J Clin Nutr. 1977;30:1603–11. doi: 10.1093/ajcn/30.10.1603. [DOI] [PubMed] [Google Scholar]
- 29.Hyldstrup L, Andersen T, McNair P, Breum L, Transbol I. Bone metabolism in obesity: changes related to severe overweight and dietary weight reduction. Acta Endocrinol (Copenh) 1993;129:393–8. doi: 10.1530/acta.0.1290393. [DOI] [PubMed] [Google Scholar]
- 30.Svendsen OL, Hassager C, Christiansen C. Effect of an energy-restrictive diet, with or without exercise, on lean tissue mass, resting metabolic rate, cardiovascular risk factors, and bone in overweight post-menopausal women. Am J Med. 1993;95:131–40. doi: 10.1016/0002-9343(93)90253-l. [DOI] [PubMed] [Google Scholar]
- 31.Snijder MB, van Dam RM, Visser M, et al. Adiposity in relation to vitamin D status and parathyroid hormone levels a population-based study in older men and women. J Clin Endocrinol Metab. 2005;90:4119–23. doi: 10.1210/jc.2005-0216. [DOI] [PubMed] [Google Scholar]
- 32.Brouwer DA, van Beek J, Ferwerda H, et al. Rat adipose tissue rapidly accumulates and slowly releases an orally-administered high vitamin D dose. Br J Nutr. 1998;79:527–32. doi: 10.1079/bjn19980091. [DOI] [PubMed] [Google Scholar]
- 33.Sandler RB. Muscle strength and skeletal competence: implications for early prophylaxis. Calcif Tissue Int. 1988;42:281–3. doi: 10.1007/BF02556359. [DOI] [PubMed] [Google Scholar]
- 34.Shapses SA, Heshka S, Heymsfield SB. Effect of calcium supplementation on weight and fat loss in women. J Clin Endocrinol Metab. 2004;89:632–7. doi: 10.1210/jc.2002-021136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zemel MB. Calcium and dairy modulation of obesity risk. Obes Res. 2005;13:192–3. doi: 10.1038/oby.2005.26. [DOI] [PubMed] [Google Scholar]
- 36.Lanou AJ. Data do not support recommending dairy products for weight loss. Obes Res. 2005;13:191. doi: 10.1038/oby.2005.25. letter. [DOI] [PubMed] [Google Scholar]
- 37.Laskey MA, Lyttle KD, Flaxman ME, Barber RW. The influence of tissue depth and composition on the performance of the Lunar dual-energy X-ray absorptiometer whole-body scanning mode. Eur J Clin Nutr. 1992;46:39–45. [PubMed] [Google Scholar]
- 38.Prentice A, Parsons TJ, Cole TJ. Uncritical use of bone mineral density in absorptiometry may lead to size-related artifacts in the identification of bone mineral determinants. Am J Clin Nutr. 1994;60:837–42. doi: 10.1093/ajcn/60.6.837. [DOI] [PubMed] [Google Scholar]
- 39.Blake GM, McKeeney DB, Chhaya SC, Ryan PJ, Fogelman I. Dual energy x-ray absorptiometry: the effects of beam hardening on bone density measurements. Med Phys. 1992;19:459–65. doi: 10.1118/1.596834. [DOI] [PubMed] [Google Scholar]
- 40.Svendsen OL, Hendel HW, Gotfredsen A, Pedersen BH, Andersen T. Are soft tissue composition of bone and non-bone pixels in spinal bone mineral measurements by DXA similar? Impact of weight loss. Clin Physiol Funct Imaging. 2002;22:72–7. doi: 10.1046/j.1475-097x.2002.00398.x. [DOI] [PubMed] [Google Scholar]
- 41.Vestergaard P, Borglum J, Heickendorff L, Mosekilde L, Richelsen B. Artifact in bone mineral measurements during a very low calorie diet: short-term effects of growth hormone. J Clin Densitom. 2000;3:63–71. doi: 10.1385/jcd:3:1:063. [DOI] [PubMed] [Google Scholar]
- 42.Tothill P, Pye DW. Errors due to non-uniform distribution of fat in dual X-ray absorptiometry of the lumbar spine. Br J Radiol. 1992;65:807–13. doi: 10.1259/0007-1285-65-777-807. [DOI] [PubMed] [Google Scholar]
- 43.Prevrhal S, Lu Y, Genant HK, Toschke JO, Shepherd JA. Towards standardization of dual X-ray absorptiometry (DXA) at the forearm: a common region of interest (ROI) improves the comparability among DXA devices. Calcif Tissue Int. 2005;76:348–54. doi: 10.1007/s00223-004-0050-z. [DOI] [PubMed] [Google Scholar]
- 44.Kiebzak GM, Lewiecki EM, Petak SM. Impact of using the ultradistal radius region of interest on diagnostic classification. J Clin Densitom. 2004;7:143–52. doi: 10.1385/jcd:7:2:143. [DOI] [PubMed] [Google Scholar]
- 45.Schneider P, Borner W, Rendl J, Eilles C, Schlisske K, Scheubeck M. Significance of 2 different bone density measurement methods in the assessment of mineral content of the peripheral and axial skeleton. Z Orthop Ihre Grenzgeb. 1992;130:16–21. doi: 10.1055/s-2008-1039507. in German. [DOI] [PubMed] [Google Scholar]