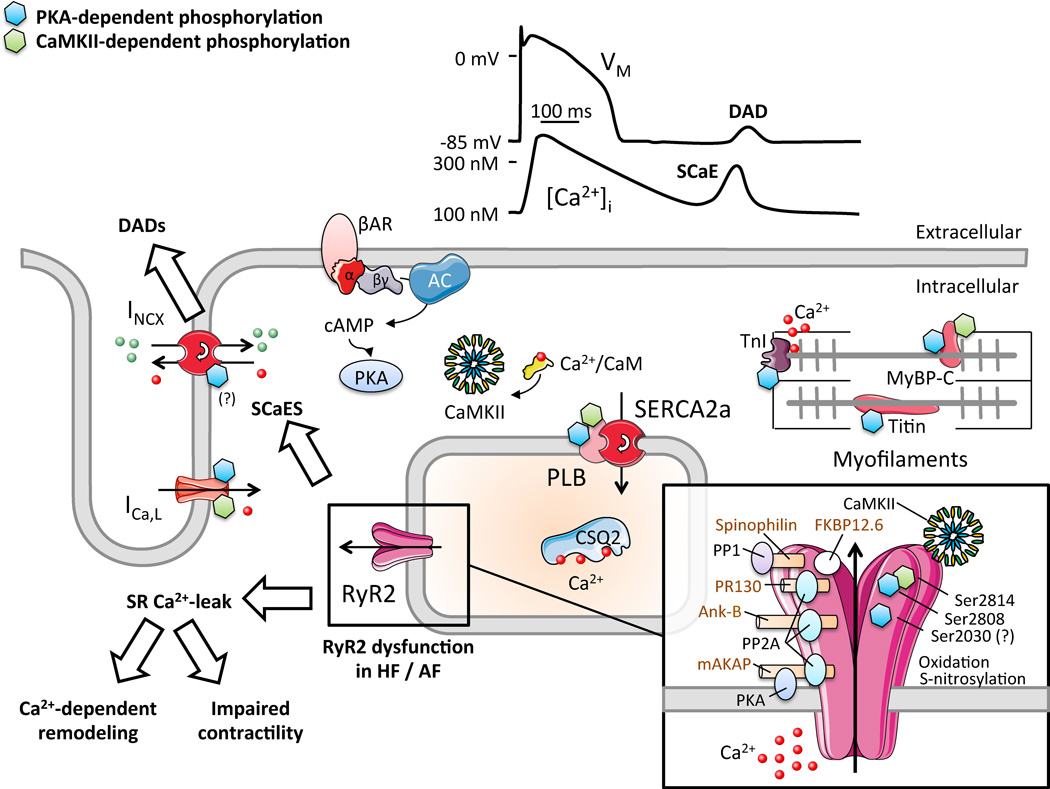
Figure 2.
Schematic representation of the key determinants of excitation-contraction coupling in cardiomyocytes. Physiologicallly, Ca2+-entry through L-type Ca2+-channels (ICa,L) triggers SR Ca2+-release through RyR2. The systolic Ca2+-transient activates myofilaments, initiating contraction. Diastolic relaxation occurs when Ca2+ is transported into SR via SERCA2a and out of the myocyte via NCX1. In heart failure (HF) and atrial fibrillation (AF), altered RyR2-phosphorylation increases SR Ca2+-leak, promotes Ca2+-dependent remodeling and impairs contractility. Spontaneous SR Ca2+-release events (SCaEs) promote delayed afterdepolarizations (DADs) and triggered activity. Inset shows RyR2-macromolecular complex with accessory proteins, protein kinases and phosphatases (and their respective anchoring proteins) that control phosphorylation levels. Protein kinase-A (PKA) and Ca2+-calmodulin-dependent protein kinase-II (CaMKII)-dependent phosphorylation sites are indicated with blue and green “P” symbols, respectively.