Figure 2.
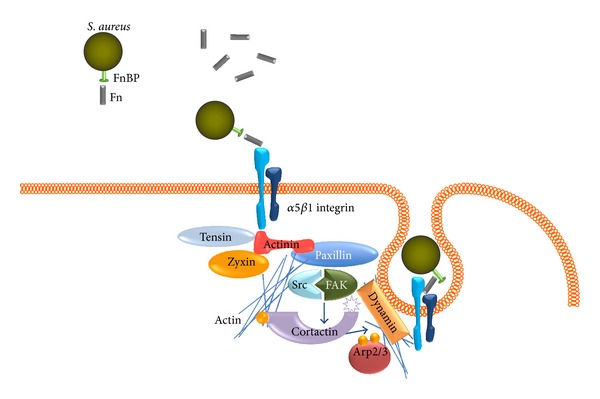
Summary of α5β1 integrin-mediated internalization of S. aureus into NPPCs. The RGD motif in fibronectin (Fn) is the crucial attachment site for fibronectin receptors, such as integrins. The activation and clustering of α5β1 integrin trigger particular signaling pathways and the accumulation of a focal adhesion-like protein complex in the vicinity of attached bacteria, as characterized by the recruitment of actinin, paxillin, zyxin, tensin, focal adhesion kinase (FAK), and Src kinase [32–34]. A crucial step in these signaling events is the reorganization of the actin cytoskeleton. Cortactin, an actin-binding protein, has been identified as one of the effectors of activated FAK and Src kinases, which associates with Arp2/3 complex to promote actin polymerization and binds to dynamin-2, a regulator of endocytosis [33, 35, 36].
