Abstract
The transformable cyanobacterium, Synechococcus sp. PCC7942, was used to study the genetics of resistance to the herbicide diuron. In wild-type cells, diuron binds to one of the core proteins, called D1, of photosystem II reaction centres. This binding prevents the transfer of electrons from QA, the primary quinone acceptor, to QB, which is necessary to create the charge separation that drives ATP synthesis. A single amino acid substitution in the D1 protein reduces diuron binding and confers herbicide resistance to reaction centres containing the substituted D1 protein. In Synechococcus 7942, the D1 protein is encoded by three functional genes called psbAI, psbAII and psbAIII. By selectively altering one member at a time of the three-member psbA gene family, we have demonstrated that diuron-resistant alleles are dominant to diuron-sensitive alleles. The relative abundance of the different psbA gene transcripts is correlated with the fraction of diuron-resistant reaction centres and with the degree of diuron resistance. Growth in sublethal diuron selectively increases the steady-state levels of transcripts of genes (psbA and psbD) encoding the core proteins of photosystem II. We have also found that turnover of the D1 protein can be uncoupled from electron transport through photosystem II.
Keywords: cyanobacteria, diuron, D1 turnover, herbicide resistance, photosystem II
Full text
PDF


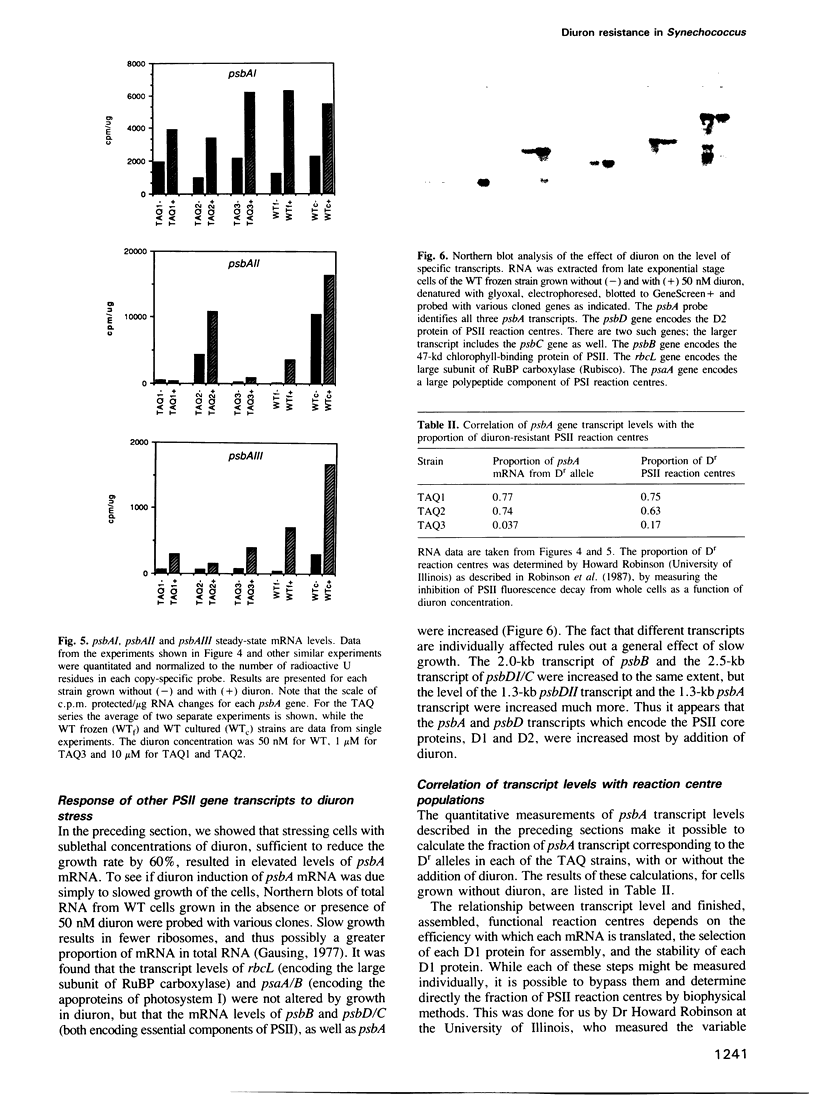
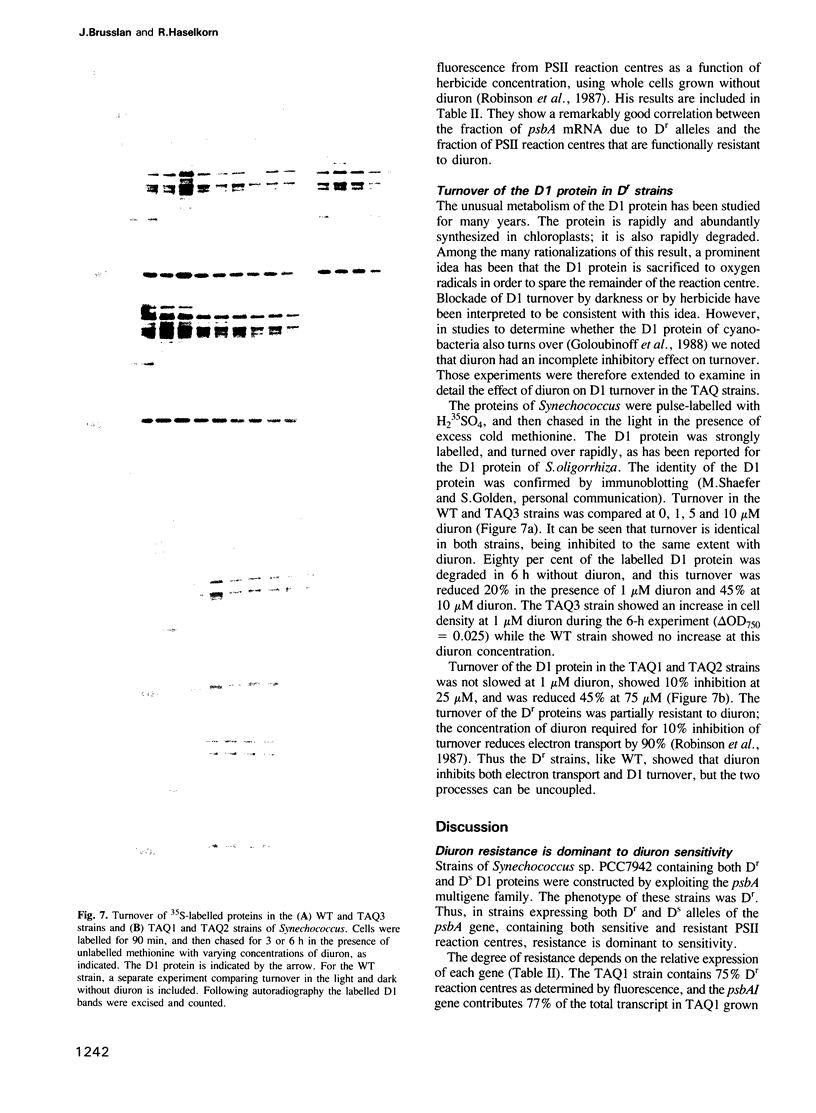

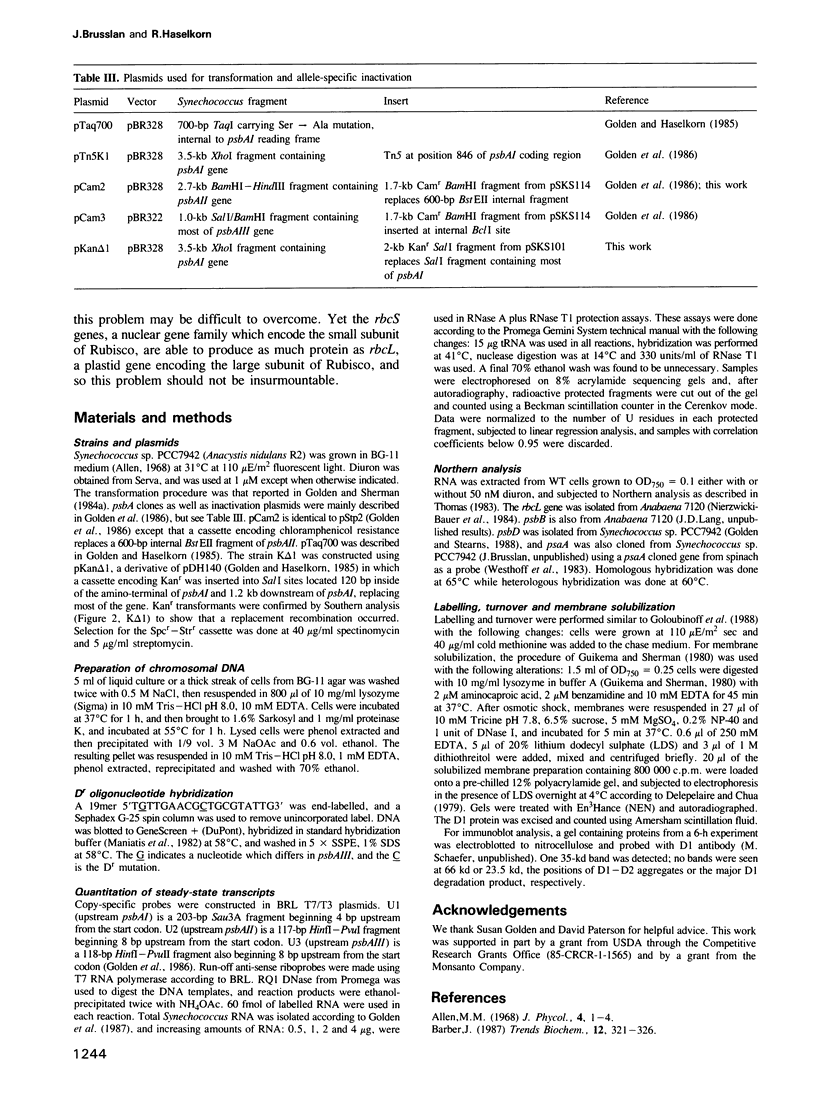

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boynton J. E., Gillham N. W., Harris E. H., Hosler J. P., Johnson A. M., Jones A. R., Randolph-Anderson B. L., Robertson D., Klein T. M., Shark K. B. Chloroplast transformation in Chlamydomonas with high velocity microprojectiles. Science. 1988 Jun 10;240(4858):1534–1538. doi: 10.1126/science.2897716. [DOI] [PubMed] [Google Scholar]
- Cheung A. Y., Bogorad L., Van Montagu M., Schell J. Relocating a gene for herbicide tolerance: A chloroplast gene is converted into a nuclear gene. Proc Natl Acad Sci U S A. 1988 Jan;85(2):391–395. doi: 10.1073/pnas.85.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delepelaire P., Chua N. H. Lithium dodecyl sulfate/polyacrylamide gel electrophoresis of thylakoid membranes at 4 degrees C: Characterizations of two additional chlorophyll a-protein complexes. Proc Natl Acad Sci U S A. 1979 Jan;76(1):111–115. doi: 10.1073/pnas.76.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson J. M., Rahire M., Rochaix J. D., Mets L. Herbicide resistance and cross-resistance: changes at three distinct sites in the herbicide-binding protein. Science. 1985 Apr 12;228(4696):204–207. doi: 10.1126/science.228.4696.204. [DOI] [PubMed] [Google Scholar]
- Gaba V., Marder J. B., Greenberg B. M., Mattoo A. K., Edelman M. Degradation of the 32 kD Herbicide Binding Protein in Far Red Light. Plant Physiol. 1987 Jun;84(2):348–352. doi: 10.1104/pp.84.2.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gausing K. Regulation of ribosome production in Escherichia coli: synthesis and stability of ribosomal RNA and of ribosomal protein messenger RNA at different growth rates. J Mol Biol. 1977 Sep 25;115(3):335–354. doi: 10.1016/0022-2836(77)90158-9. [DOI] [PubMed] [Google Scholar]
- Golden S. S., Brusslan J., Haselkorn R. Expression of a family of psbA genes encoding a photosystem II polypeptide in the cyanobacterium Anacystis nidulans R2. EMBO J. 1986 Nov;5(11):2789–2798. doi: 10.1002/j.1460-2075.1986.tb04569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden S. S., Brusslan J., Haselkorn R. Genetic engineering of the cyanobacterial chromosome. Methods Enzymol. 1987;153:215–231. doi: 10.1016/0076-6879(87)53055-5. [DOI] [PubMed] [Google Scholar]
- Golden S. S., Haselkorn R. Mutation to herbicide resistance maps within the psbA gene of Anacystis nidulans R2. Science. 1985 Sep 13;229(4718):1104–1107. doi: 10.1126/science.3929379. [DOI] [PubMed] [Google Scholar]
- Golden S. S., Sherman L. A. Optimal conditions for genetic transformation of the cyanobacterium Anacystis nidulans R2. J Bacteriol. 1984 Apr;158(1):36–42. doi: 10.1128/jb.158.1.36-42.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden S. S., Stearns G. W. Nucleotide sequence and transcript analysis of three photosystem II genes from the cyanobacterium Synechococcus sp. PCC7942. Gene. 1988 Jul 15;67(1):85–96. doi: 10.1016/0378-1119(88)90011-x. [DOI] [PubMed] [Google Scholar]
- Greenberg B. M., Gaba V., Mattoo A. K., Edelman M. Identification of a primary in vivo degradation product of the rapidly-turning-over 32 kd protein of photosystem II. EMBO J. 1987 Oct;6(10):2865–2869. doi: 10.1002/j.1460-2075.1987.tb02588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschberg J., McIntosh L. Molecular Basis of Herbicide Resistance in Amaranthus hybridus. Science. 1983 Dec 23;222(4630):1346–1349. doi: 10.1126/science.222.4630.1346. [DOI] [PubMed] [Google Scholar]
- Kyle D. J., Ohad I., Arntzen C. J. Membrane protein damage and repair: Selective loss of a quinone-protein function in chloroplast membranes. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4070–4074. doi: 10.1073/pnas.81.13.4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattoo A. K., Hoffman-Falk H., Marder J. B., Edelman M. Regulation of protein metabolism: Coupling of photosynthetic electron transport to in vivo degradation of the rapidly metabolized 32-kilodalton protein of the chloroplast membranes. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1380–1384. doi: 10.1073/pnas.81.5.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nierzwicki-Bauer S. A., Curtis S. E., Haselkorn R. Cotranscription of genes encoding the small and large subunits of ribulose-1,5-bisphosphate carboxylase in the cyanobacterium Anabaena 7120. Proc Natl Acad Sci U S A. 1984 Oct;81(19):5961–5965. doi: 10.1073/pnas.81.19.5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohad I., Kyle D. J., Hirschberg J. Light-dependent degradation of the Q(B)-protein in isolated pea thylakoids. EMBO J. 1985 Jul;4(7):1655–1659. doi: 10.1002/j.1460-2075.1985.tb03833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA transferred or dotted nitrocellulose paper. Methods Enzymol. 1983;100:255–266. doi: 10.1016/0076-6879(83)00060-9. [DOI] [PubMed] [Google Scholar]
- Weinbaum S. A., Gressel J., Reisfeld A., Edelman M. Characterization of the 32,000 Dalton Chloroplast Membrane Protein: III. Probing Its Biological Function in Spirodela. Plant Physiol. 1979 Nov;64(5):828–832. doi: 10.1104/pp.64.5.828. [DOI] [PMC free article] [PubMed] [Google Scholar]