Abstract
There is growing evidence that the commensal bacteria in the gastrointestinal tract (the gut microbiota) influence the development of autoimmunity in rodent models. Since humans have co-evolved with commensals for millennia, it is likely that people, who are genetically predisposed to autoimmunity, harbor gut microbial communities that similarly influence the onset and/or severity of disease. Beyond the current efforts to identify such disease-promoting or –preventing commensals (‘pathobionts’ or ‘symbionts’), it will be important to determine what factors modulate them. Dietary changes are known to affect both the composition and function of the gut microbial communities, which in turn can alter the innate and adaptive immune system. In this review, we focus on the relationships between diet, microbiota, and autoimmune diseases. We hypothesize that the beneficial and life-prolonging effects of caloric restriction on a variety of autoimmune models including lupus might partly be mediated by its effects on the gut microbiome and associated virome, the collection of all viruses in the gut. We give recent examples of the immunomodulatory potential of select gut commensals and their products or diet-derived metabolites in murine models of arthritis, multiple sclerosis and type 1 diabetes. Lastly, we summarize the published phenotypes of germ-free mouse models of lupus and speculate on any role of the diet-sensitive microbiome and virome in systemic lupus and the related antiphospholipid syndrome.
Keywords: Microbiome, virome, caloric restriction, autoimmunity, molecular mimicry
Introduction
Humans have co-evolved with a variety of microorganisms for millenia. This has led to remarkable co-dependence and blurs the distinction between self and non-self. Mitochondria, for instance, are thought to have evolved from an extreme symbiosis with certain prokaryotes. Similarly, endogenous retroviruses have integrated into the host’s genome and are passed on through generations. On the other end of the spectrum are infectious agents that chronically colonize a host with persistent inflammation since the host/microbe interactions have not resulted in homeostasis, aptly called “frustrated commensalism”.1 Various microorganisms inhabit our bodies, ranging from protozoa, eukaryotic fungi, bacteria and archaea to viruses. Advances in culture-independent technologies to enumerate the microbiota (in particular next-generation sequencing of 16S ribosomal RNA) have elucidated that humans are colonized with dozens of bacterial species in the stomach, hundreds on skin and oral mucosa, and thousands within the gut. The Human Microbiome Project has recently completed the first comprehensive characterization of these microbiomes in healthy human subjects.2 Among the sampled sites, the gastrointestinal tract is by far the largest reservoir for commensal bacteria and is profoundly influenced by diet. Dietary changes are known to affect both the composition and function of the gut microbial communities, which in turn can modulate the host’s innate and adaptive immune system.3 In this review, we focus on the relationships between diet, microbiota, and autoimmune diseases.
Diet and gut microbial communities
The nutritional value of food is partly derived from the composition and function of a consumer’s gut microbial composition. Vice versa, food significantly changes the composition of the gut microbiota and its genetic makeup (the gut microbiome).4, 5 Comparison of the fecal microbiomes of obese and lean humans as well as monocygotic and dizygotic twin pairs concordant for leanness or obesity allowed for deep insights into this inter-relationship.6, 7 Remarkably, the human microbiota of an obese host that was found to be markedly different from that of a lean host, were able to induce weight gain when transplanted into germ-free mice.6 The phylogenetic composition of the microbiota in lean versus obese individuals has also been examined in several other studies.8 In addition, comparisons of fecal samples and diets from mammals living in different habitats provided insights into the factors driving the evolution of the gut microbiome.5, 9 Taken together, these large-scale sequencing efforts support the notion that differences in diet dictate the development and composition of the gut microbiota. Studies using humanized, gnotobiotic mice (germ-free mice colonized with human microbiota) then allowed for testing specific mechanisms that drive the diet-host-gut microbial community interactions.4, 10
While such elegant studies have started to explain basic mechanisms of how human microbial communities lead to obesity and metabolic disease, little is known about the interplay of diet and gut microbiota in human immune-mediated diseases. Caloric restriction, for instance, is extremely well studied with regards to host immune effects but the impact of caloric restriction on the gut microbiome has been neglected thus far. The caloric restriction-induced alterations on the host’s immune system are manifold11 and the host’s molecular signaling pathways are in depth dissected, e.g. the sirutin and mammalian target of rapamycin (mTOR) pathways.12, 13 It is, however, unclear if these are always the direct consequence of caloric restriction or indirectly mediated via modulation of gut commensals. It is possible that the protective effects are partly conferred by alterations in gut microbial communities since the intestinal epithelium that is heavily colonized by the microbiota also changes with caloric restriction.14 The epithelial effects appear to be mediated via inhibition of mTOR complex 1 (mTORC1) which in turn augments Paneth cell function.15 This leads to proliferation of intestinal stem cells coupled with slowing of enterocyte differentiation and subsequently decreased villus size. These events could theoretically induce adaptive changes in the epithelia-colonizing commensals.
In addition, Li et al. demonstrated how diet directly impacts immunity via a plant-derived nutrient (the so called indole-3-carbinol) that is found in broccoli, cabbage and cauliflower. A metabolite of this phytochemical profoundly shapes intestinal immune responses by binding and activating the so called aryl hydrocarbon receptor.16 This receptor is expressed at high levels on intraepithelial lymphocytes and epithelial cells. Importantly, Li et al. switched the animal diet from a standard murine diet to a synthetic feed that lacks ingredients of vegetable origin. The modified diet led to a decline in intraepithelial lymphocyte numbers, enhanced susceptibility to epithelial damage, and increased numbers of intestinal Bacteroides, a Gram-negative genus prevalent in mammalian intestines that is able to induce colitis in a host-genotype-specific manner.16, 17 Dietary changes can therefore profoundly affect the composition of gut microbial communities as discussed also elsewhere.3 While diet-microbiota interactions are little studied in autoimmune diseases, direct effects of the gut microbiota on autoimmunity are emerging from several murine studies and are summarized next.
Microbiota and autoimmune diseases
The role of gut commensals in models of autoimmune disease
The capacity of the intestinal microbiota to shape immune responses outside the intestine will be highlighted with examples from one potently immunomodulatory species, the unculturable, Gram-positive, spore-forming segmented filamentous bacteria (SFB), which are influenced by a single micronutrient, vitamin A.3 SFB support the development of autoimmune arthritis18 and experimental autoimmune encephalomyelitis,19 while being involved in protection from type 1 diabetes.20 In all of these models, the immunologic phenotypes induced by SFB have been linked to excessive T helper 17 (Th17) cell responses consistent with the Th17-inducing function of SFB, which was demonstrated by Littman and colleagues in non-autoimmune mice.21
However, the cellular and molecular mechanisms by which intestinal commensals influence autoimmune responses at distal sites remain poorly understood. One of the first mouse models that shed more light on these mechanisms is the K/BxN T cell receptor (TCR) transgenic mouse model. In this model, the initiation phase is predominantly mediated by the adaptive immune system, which culminates in autoantigen-antibody immune complex deposition on synovial surfaces and subsequent activation of innate immune mechanisms leading to erosive polyarthritis. Strikingly, germ-free K/BxN mice develop only an attenuated form of autoimmune arthritis, as well as a decrease in autoantibodies, germinal centers, and splenic Th17 cells.18 The phenotype was also characterized by a loss of small intestinal lamina propria Th17 cells. Importantly, SFB alone could induce Th17 cells, autoantibodies, and arthritis in this model, supporting a causal role for these commensal bacteria in erosive polyarthritis resembling histologically human rheumatoid arthritis.
Examining the role of the microbiota on the onset and severity of experimental autoimmune encephalitis, a model for multiple sclerosis, Mazmanian and colleagues demonstrated that a germ-free state reduced inflammation and clinical pathology compared with colonized mice19 as also shown more recently in another model for multiple sclerosis.22 Dendritic cells (DCs) from germ-free animals cannot prime autoantigen-specific T cell activation as efficiently as DCs from colonized animals. Remarkably, SFB-monocolonized animals (germ-free animals that were colonized only with SFB) showed increased encephalitogenic Th17 cells not only in the gut but also within the spinal cords. Thus, a single commensal microbe, via its ability to promote a specific T helper cell subset, can drive both joint and brain inflammation in two different models.
While these autoimmune diseases are mitigated in the germ-free state, others are exacerbated suggesting different mechanisms at play as, for instance, in type 1 diabetes. Consistent with the ‘hygiene hypothesis’, the incidence of type 1 diabetes is higher in countries with stricter hygienic practices23, 24 and also higher in cleaner colonies of the non-obese diabetic (NOD) mouse, a prototypical model for this autoimmune disease.25 Further support of a protective role for the gut microbiota in type 1 diabetes comes from studies of MyD88-knockout NOD mice.26 MyD88 (or myeloid differentiation primary response gene 88) is an innate immune system signaling molecule downstream of toll-like receptor (TLR) and interleukin-1/-18 receptor signaling. Genetic deletion of MyD88 in NOD mice leads to an altered composition of the gut microbiota and prevents the development of type 1 diabetes under normal housing conditions.26 Importantly, the protective effect was attributed to commensals otherwise kept in check by MyD88 signaling, because mice expressing or not expressing this molecule were equally susceptible to disease when housed in germ-free isolators.26 In an attempt to identify protective commensals in this model, segregation studies with naturally transmitted commensals were performed.20 Interestingly, when tracking the incomplete penetrance of SFB colonization of NOD mice due to admixture of SFB-devoid NOD mice from a commercial vendor, segregation of SFB colonization around 6 weeks of age was correlated with diabetes protection despite the pathogenic potential of SFB in the multiple sclerosis and arthritis models described above. It remains to be shown whether SFB act in concert with other protective commensals or alone, but this study suggests that the same commensal can act either as a beneficial symbiont or a disease-promoting pathobiont depending on the autoimmune disease (mediated by different effector mechansims) and the gut microbiota composition. Surprisingly, male NOD mice remained relatively disease-free in this model regardless of the SFB colonization status suggesting a commensal-dependent sexual dimorphism.20 Indeed, sex differences in the gut microbiota are responsible for hormone-dependent autoimmunity in NOD mice.27 While type 1 diabetes in humans is not female-biased, the NOD mouse is also a model for female-biased Sjogren’s syndrome and autoimmune polyglandular syndrome type II manifesting with thyroiditis and adrenalitis. Sex-specific commensals might thus play a role in these and possibly other female-biased autoimmune diseases like systemic lupus erythematosus (SLE) and antiphospholipid syndrome (APS) discussed below.
Collectively, these studies suggest that the microbiota contains organisms that direct both pro- and anti-inflammatory immune responses depending on the immunologic context, the genetic makeup and sex of the host, and likely also the overall microbial community structure.
Commensal products affecting autoimmunity
We have discussed thus far only the effects of colonization with whole, intact commensals. Which microbial structures or mechanisms are mediating the profound immunologic effects of SFB remain to be determined for the vast majority of commensals. In some instances, progress has been made in defining specific molecules that mediate immunomodulatory effects. Oral administration of the capsular polysaccharide A of the human gut commensal Bacteroides fragilis can protect against experimental autoimmune encephalomyelitis in mice via conversion of naïve CD4+ T cells into interleukin (IL)-10-producing FoxP3+ regulatory T (Treg) cells.28 This effect appears to be mediated by Treg-enhancing dendritic cells that accumulate in the cervical lymph node of polysaccharide A-treated animals. Protection is critically dependent on IL-10 since induction of encephalomyelitis in IL-10−/− mice was not inhibited by oral administration of polysaccharide A. Short-chain fatty acids, which are generated by fermentation of dietary fiber by the intestinal microbiota, are an example of diet-derived bacterial products that affect immune function. A reduction in short-chain fatty acids has long been associated with inflammatory bowel disease. Short-chain fatty acids bind the G-protein-coupled receptor 43 (GPR43) and activation of GPR43 by short-chain fatty acids is necessary for physiologic resolution of inflammation, since GPR43-deficient (Gpr43−/−) mice showed exacerbated or unresolving inflammation in models of colitis, arthritis and asthma.29 Germ-free mice, which express little or no bacteria-derived short-chain fatty acids, showed a similar dysregulation of inflammatory responses. GPR43 binding of short-chain fatty acids thus provides a molecular link between diet, gut commensal metabolism, and inflammatory responses.
Is there a role for the gut microbiome/virome in systemic lupus erythematosus?
The evidence that the intestinal microbiota is involved in the development of systemic autoimmunity, in particular SLE, is less clear than for the autoimmune diseases discussed above. The onset and/or severity of experimental models of lupus-like disease are not profoundly altered when comparing germ-free and conventionally raised mice (see Table 1 for a comparison of published phenotypes).30-35 The select germ-free models that were studied mostly in the 1960s and 70s, however, might not represent all mechanisms involved in SLE and were not followed up with antibiotic treatments to circumvent the disturbed developmental processes that are known to exist in a permanent germ-free state. More recent discoveries of an important role of TLR7/9 in the pathogenesis of lupus36 suggest that bacterial or viral commensal triggers might also contribute to SLE pathogenesis. Interestingly, TLR9 engagement by commensal DNA was shown to modulate the effector/regulatory T cell equilibrium in non-autoimmune mice.37 These findings raise the possibility that commensals and their nucleic acids might affect immunoregulatory pathways leading to systemic autoimmunity.
Table I.
Summary of the germ-free phenotypes of spontaneous or inducible models of lupus-like disease.a
| References | Strain/Model | Autoimmune Serologies | Spleen | Kidneys | Other Manifestations/Findings |
|---|---|---|---|---|---|
| East et at29 | |||||
| NZB GF | Coombs+ at 8-11mo. ANA+ (6 mo.): 25% |
8 - 11 mo.: Enlarged, thin, tough, dark purple |
Histology N/A | Ceca: enlarged, distended Superficial LNs: small, difficult to detect Virus-like particles: at 6, 8, 11 mo. Antibodies to polyoma virus: not detected |
|
| NZB Conv | Coombs+ at 4-6 mo. ANA+ (>6 mo.): 20-30% |
8 - 11 mo.: Enlarged, thick, spongy, pink-red |
Histology N/A | Ceca: not detailed Superficial LNs: prominent, palpable Virus-like particles: at 1week-14 mo. Antibodies to polyoma virus: detectable |
|
|
Barnes &
Tuffrey27 |
|||||
| NZB GF | Coombs+: N/A ANA+ (>5 mo.) 50% |
Not Studied | Not Studied | Thymus (post-mortem): similar to Conv NZB | |
| NZB Conv | Coombs+ at 4 mo. ANA+ (5-6 mo.): 22% |
Not Studied | Not Studied | Thymus (post mortem): germinal follicle formation and accumulation of plasma cells |
|
|
East &
Branca28 |
|||||
| NZB GF | Coombs+ unequivocal at 10-17 mo. |
Splenomegaly (later onset): average wt 0.51g(0.1-0.7g); smaller germinal centers at 8 mo. |
Glomerulonephritis present (at 8 mo.: enlarged glomeruli, mesangial proliferation) |
Thymus (6-10 mo.): larger than Conv Inguinal LN (8 mo.): involuted Mesenteric LN (8 mo.): smaller, lymphocyte depleted Anemia: less severe at 9-10 mo. (hematocrit 32-40%) |
|
| NZB Conv | Coombs+ “earlier” than GF |
Splenomegaly; average wt 1.26g (0.4-2.3g) |
Glomerulonephritis present (severity not detailed) |
Thymus (6-10 mo.): smaller than GF (not detailed) Inguinal LN (8 mo.): large, active follicles Mesenteric LN (8 mo.): larger (not detailed) Anemia: severe at 9-10 mo. (hematocrit 17-38%) |
|
| Unni et at32 | |||||
| NZB GF | Coombs+ “no significant difference” to Conv (but lower incidence at 6 mo. with 0-20% depending on sex) ANA+ at 6 mo. (also higher than Conv at 9 and 12 mo.; p<0.025) |
N/A | Decreased incidence and severity of glomerular lesions and lymphocytic infiltrates than Conv (at 12 mo.; p<0.005) |
Ceca: dilated at 6-12 mo. Liver (12 mo.): lower incidence of periportal infiltrate (8 of 27 mice) Lung (12 mo.): lower incidence of lymphocytic infiltrates (8 of 27 mice) γ-globulins lower than Conv in all age groups (p<0.01) |
|
| NZB Conv | Coombs+ at 6 mo. 40- 60% ANA+ at 12 mo. |
N/A | Progressive glomerular lesions and lymphocytic infiltrates (greater than GF at 12 mo.); greater proteinuria |
Ceca: not detailed Liver (12 mo.): frequent periportal infiltrates (31 of 39 mice) Lungs (12 mo.): frequent lymphocytic infiltrates (32 of 39 mice) γ-globulins higher than GF at 3, 6, 9, 12 mo. |
|
|
Maldonado
et al30 |
|||||
| MRL/MpJ- Faslpr GF |
Trend towards higher IgG2a and anti-Sm at 5 mo. Anti-dsDNA+: 89% |
No difference in wt at 5 mo. |
Similar glomerulonephritis and proteinuria at 5 mo. (with antigen-free diet: little glomerular disease, little vascular/interstitial changes, minimal C3 deposits, little proteinuria) |
Ceca: enlarged (but mitigated if fed with antigen-free diet) Ear lesions: absent (but present in 7 of 8 females and 1 of 9 males fed with antigen-free diet) LN: No difference in LN T cell subsets and extensive lymphadenopathy (LN weight from male mice fed with antigen-free diet was lower than that of GF mice fed with natural ingredients) |
|
| MRL/MpJ- Faslpr Conv |
Higher IgM (p=0.05) and anti-ssDNA (p=0.04) at 5 mo. Anti-dsDNA+: 43% |
Same spleen wt at 5 mo. as GF |
Similar glomerulonephritis and proteinuria at 5 mo. as GF |
Ceca: normal size (not detailed) Ear lesions: “typical for MRL-lpr” (not detailed) LN: extensive lymphadenopathy; same LN T cell subsets as in GF |
|
| Mizutani et al31 | |||||
| BALB/c GF treated with i.p. pristane |
Anti-RNP/Smith IgG at 6 mo. post-treatment lower than Conv (p<0.000l) |
Germinal center formation “at low frequency” lymphoid follicles poorly developed |
Histology: N/A | Ceca: enlarged (in all 62 mice) Pristane-induced IL-6, IL-12, hyper-γ-globulinemia detectable (no comparison made to Conv) |
|
| BALB/c Conv treated with i.p. pristane |
Anti-RNP/Smith IgG at 6 mo. higher than GF (but similar levels of anti-Su) |
Formation of germinal centers and lymphoid follicles (not detailed) |
Histology: N/A | Ceca: normal size (not detailed) Pristane-induced IL-6, IL-12, hyper-γ-globulinemia not detailed |
Comparison of published germ-free (GF) versus conventionally raised (Conv) mice that develop spontaneous or inducible features of systemic, lupus-like autoimmunity. Cecal size (if reported) is listed under “Other Manifestations/Findings” as an indirect measure of a successful GF state. Abbreviations: GF, germ-free; Conv, conventional; ANA, antinuclear antibodies; N/A, not assessed; mo., months; LN, lymph nodes; wt, weight; i.p., intraperitoneal.
In addition, several dietary manipulations can alter the course of SLE, which may be partly mediated by effects on the gut microbiota as hypothesized above. Studies have shown that caloric restriction prevents the progression of lupus-like disease in NZB and (NZBxNZW)F1 mice38, 39 as well as the SLE-associated antiphospholipid syndrome (APS) in (NZWxBXSB)F1 mice.40 Other dietary interventions or factors, such as polyunsaturated fatty acids, vitamins A, D, and E, and phytoestrogens also lead to improved outcome in animal models of SLE, mostly via reduction in proteinuria and glomerulonephritis, as summarized elsewhere.41 Furthermore, using two isocaloric diets that differed in their fat composition, Reifen et al showed that enrichment with n-3 polyunsaturated fatty acids prevents fetal loss and other clinical manifestations of lupus-associated APS.42 Taken together, these studies demonstrate a broad influence of diet on SLE and related APS. It remains to be shown whether the gut microbiota has also an impact on these diseases, and if so, which mechanisms might be at play. In APS, there is already evidence that molecular mimicry by certain pathogens can induce autoantibody production.43 It is tempting to speculate that chronic cross-reactivity with gut commensals might sustain autoantibody levels in APS and perhaps other autoimmune diseases as well. We have set out to test this hypothesis in a microbiome pilot trial involving APS patients (ClinicalTrials.gov identifier NCT01787305).
The virome: crosstalk with commensal bacteria
The gut microbiota interacts not only with the host but also other organisms and environmental factors. Exogenous viruses and the virome - the genomes of all the viruses that inhabit a host - are interacting with the gut microbiota (for review see references44, 45). The RNA and DNA viruses within a mammalian host can live both within eukaryotic and prokaryotic cells, such as the gut commensal bacteria. The common ‘interferon-α (IFN-α) signature’ in the peripheral blood of SLE patients suggests a potential viral trigger of flares and represents a novel target for therapies.46 While exogenous viruses have been implicated as potential contributors (e.g. Epstein-Barr virus),47 a chronic trigger within the gastrointestinal virome or endogenous retroviruses seems at least as likely, especially since retroelements have been shown to drive autoimmunity.48 Recent studies of the intestinal virome in humans demonstrated that it is largely composed of bacteriophages.49 Importantly, the intestinal virome is also altered in response to diet changes,50 and endogenous retroviral levels are dependent on the gut microbiota and the acidity of the drinking water.51 Interestingly, crosstalk between virally stimulated type I IFNs and the innate signaling receptors NOD1/NOD2 promotes bacterial recognition in mice.52 Theoretically, similar crosstalk could exist between commensals and the virome, thereby amplifying pathogenetic events.
In summary, there are many possibilities as to how diet, microbiome and virome interactions within a host could play a role in the pathogenesis of SLE, APS, and related conditions (see Figure 1). In addition to innate signals, concomitant stimulation of adaptive immunity in a genetically predisposed host is plausible, considering the myriad of potentially cross-reactive commensals to which the host’s immune system is exposed throughout life. Interestingly, not only systemic antibody responses to gut microbes occur in healthy hosts,53 but also murine commensal-specific T cells exist in normal hosts.54 In addition, TCR cross-reactivity to environmental antigens is more common in healthy humans than previously anticipated.55 A testable hypothesis would thus be that combined innate and adaptive stimulation from certain cross-reactive commensals drive chronic autoreactivity in genetically predisposed people such as those carrying certain human leukocyte antigen (HLA) and innate immune variants (e.g. tumor necrosis factor α-induced protein 3 or TNFAIP3). Perhaps diet-related fluctuations of the gut microbiota are one of the reasons for the well-known, but unexplained, fluctuations of autoantibody levels in autoimmune patients and asymptomatic carriers.
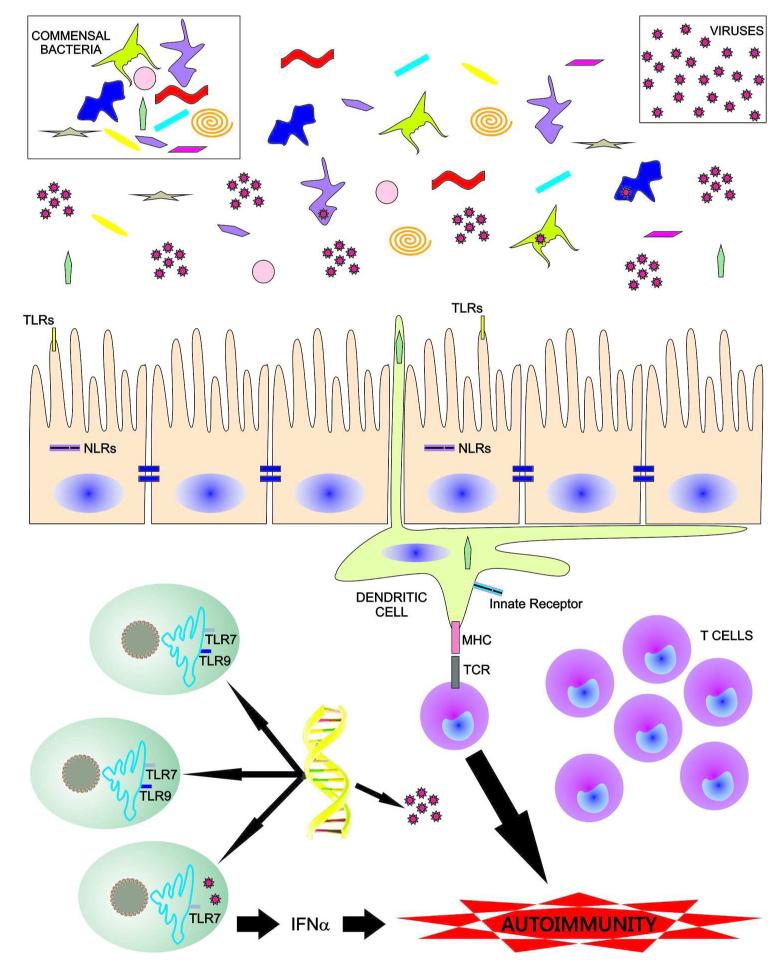
Fig. 1. Putative gut microbiome and virome interactions with an autoimune-prone host immune system.
This cartoon illustrates a hypothetical model for potential mechanisms how the gut microbiota, the gut virome and endogenous retroviruses can contribute to the pathogenesis of systemic autoimmunity. Gut commensals and viruses within the lumen of the gastrointestinal tract are influenced by diet and micronutrients (not shown). Bacteriophages within commensal bacteria are able to modify the bacteria that then affect the host by various mechanisms. Commensals are sensed by specific pattern recognition receptors on the surface or intracellular compartment of epithelial cells and resident antigen-presenting cells, e.g. toll-like receptors (TLRs) or nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs). Dendritic cells can sample commensals within the lumen and present antigens via major histocompatibility complexes (MHC) to T cell subsets that are potentially cross-reactive with self-antigens in a genetically predisposed host. Similarly, genetic predisposition to hypersensitive innate immune receptors or signaling cascades can promote an adaptive immune response to commensal and self antigens. Cross-reactive CD4+ T cells provide subsequently help to inherently autoreactive B cells (not shown) that then produce autoantibodies, leading to systemic autoimmunity. Lastly, endogenous retroviruses that have inserted into the host’s genome (shown in yellow) are also influenced by the gut microbiota and produce nucleic acids that trigger IFNα production via nucleic acid sensors like the endosomally localized TLR7. In addition, commensal DNA triggers a TLR9-mediated response in lamina propria dendritic cells (not depicted). These innate immune events may support an adaptive (auto)immune response to self structures initiated by cross-reactive commensals. See main text for references supporting several of these aspects in non-autoimmune hosts.
Acknowledgments
We would like to thank Dr. Akiko Iwasaki (Yale University) for helpful discussions on endogenous retroviruses and host immune responses. This work was supported by a grant from the National Institutes of Health (K08 AI095318 to M.A.K.), a Yale Rheumatic Diseases Research Core Center pilot grant (to M.A.K.), a Women’s Health Research at Yale pilot grant (to M.A.K.) and an NIH institutional training grant (T32 AI-007174-32 to O.E.P.).
Abbreviations
- SFB
segmented filamentous bacteria
- mTOR
mammalian target of rapamycin
- mTORC1
mTOR complex 1
- MyD88
myeloid differentiation primary response gene (88)
- NOD
non-obese diabetic mouse
- SLE
systemic lupus erythematosus
- APS
antiphospholipid syndrome
- GPR43
G-protein-coupled receptor 43
- TLR
toll-like receptor
- NOD1/NOD2
nucleotide binding oligomerization domain 1/2
- NLRs
NOD-like receptors
- IFN-α
interferon alpha
- TNFAIP3
tumor necrosis factor alpha-induced protein 3
- TCR
Tcell receptor
- MHC
major histocompatibility complex
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Nussbaum JC, Locksley RM. Infectious (Non)tolerance--frustrated commensalism gone awry? Cold Spring Harbor perspectives in biology. 2012:4. doi: 10.1101/cshperspect.a007328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Relman DA. Microbiology: Learning about who we are. Nature. 2012;486:194–5. doi: 10.1038/486194a. [DOI] [PubMed] [Google Scholar]
- 3.Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. Human nutrition, the gut microbiome and the immune system. Nature. 2011;474:327–36. doi: 10.1038/nature10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goodman AL, Kallstrom G, Faith JJ, et al. Extensive personal human gut microbiota culture collections characterized and manipulated in gnotobiotic mice. Proc Natl Acad Sci U S A. 2011;108:6252–7. doi: 10.1073/pnas.1102938108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muegge BD, Kuczynski J, Knights D, et al. Diet drives convergence in gut microbiome functions across mammalian phylogeny and within humans. Science. 2011;332:970–4. doi: 10.1126/science.1198719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turnbaugh PJ, Hamady M, Yatsunenko T, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–4. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–31. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 8.Ley RE. Obesity and the human microbiome. Current opinion in gastroenterology. 2010;26:5–11. doi: 10.1097/MOG.0b013e328333d751. [DOI] [PubMed] [Google Scholar]
- 9.Ley RE, Hamady M, Lozupone C, et al. Evolution of mammals and their gut microbes. Science. 2008;320:1647–51. doi: 10.1126/science.1155725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med. 2009;1:6ra14. doi: 10.1126/scitranslmed.3000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jolly CA. Dietary restriction and immune function. J Nutr. 2004;134:1853–6. doi: 10.1093/jn/134.8.1853. [DOI] [PubMed] [Google Scholar]
- 12.Bordone L, Guarente L. Calorie restriction, SIRT1 and metabolism: understanding longevity. Nat Rev Mol Cell Biol. 2005;6:298–305. doi: 10.1038/nrm1616. [DOI] [PubMed] [Google Scholar]
- 13.Wang R, Green DR. Metabolic checkpoints in activated T cells. Nat Immunol. 2012;13:907–15. doi: 10.1038/ni.2386. [DOI] [PubMed] [Google Scholar]
- 14.Ogura M, Ogura H, Ikehara S, Dao ML, Good RA. Decrease by chronic energy intake restriction of cellular proliferation in the intestinal epithelium and lymphoid organs in autoimmunity-prone mice. Proc Natl Acad Sci U S A. 1989;86:5918–22. doi: 10.1073/pnas.86.15.5918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yilmaz OH, Katajisto P, Lamming DW, et al. mTORC1 in the Paneth cell niche couples intestinal stem-cell function to calorie intake. Nature. 2012;486:490–5. doi: 10.1038/nature11163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y, Innocentin S, Withers DR, et al. Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell. 2011;147:629–40. doi: 10.1016/j.cell.2011.09.025. [DOI] [PubMed] [Google Scholar]
- 17.Bloom SM, Bijanki VN, Nava GM, et al. Commensal Bacteroides species induce colitis in host-genotype-specific fashion in a mouse model of inflammatory bowel disease. Cell Host Microbe. 2011;9:390–403. doi: 10.1016/j.chom.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu HJ, Ivanov II, Hattori K, Shima T, Umesaki Y, Littman DR, Benoist C, Mathis D. Gut-residing segmented filamentous bacteria can drive autoimmune arthritis via T helper 17 cells. Immunity. 2010;32:815–27. doi: 10.1016/j.immuni.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee YK, Menezes JS, Umesaki Y, Mazmanian SK. Microbes and Health Sackler Colloquium: Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.1000082107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kriegel MA, Sefik E, Hill JA, Wu HJ, Benoist C, Mathis D. Naturally transmitted segmented filamentous bacteria segregate with diabetes protection in nonobese diabetic mice. Proc Natl Acad Sci U S A. 2011;108:11548–53. doi: 10.1073/pnas.1108924108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ivanov, Atarashi K, Manel N, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–98. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berer K, Mues M, Koutrolos M, et al. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature. 2011;479:538–41. doi: 10.1038/nature10554. [DOI] [PubMed] [Google Scholar]
- 23.Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med. 2002;347:911–20. doi: 10.1056/NEJMra020100. [DOI] [PubMed] [Google Scholar]
- 24.Zipris D. Epidemiology of type 1 diabetes and what animal models teach us about the role of viruses in disease mechanisms. Clin Immunol. 2009;131:11–23. doi: 10.1016/j.clim.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 25.Pozzilli P, Signore A, Williams AJ, Beales PE. NOD mouse colonies around the world--recent facts and figures. Immunol Today. 1993;14:193–6. doi: 10.1016/0167-5699(93)90160-M. [DOI] [PubMed] [Google Scholar]
- 26.Wen L, Ley RE, Volchkov PY, et al. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature. 2008;455:1109–13. doi: 10.1038/nature07336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Markle JG, Frank DN, Mortin-Toth S, et al. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science. 2013;339:1084–8. doi: 10.1126/science.1233521. [DOI] [PubMed] [Google Scholar]
- 28.Ochoa-Reparaz J, Mielcarz DW, Wang Y, et al. A polysaccharide from the human commensal Bacteroides fragilis protects against CNS demyelinating disease. Mucosal Immunol. 2010;3:487–95. doi: 10.1038/mi.2010.29. [DOI] [PubMed] [Google Scholar]
- 29.Maslowski KM, Vieira AT, Ng A, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282–6. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barnes RD, Tuffrey M. Increased red cell breakdown in adoptively immunized young NZB mice. Nature. 1966;209:1095–9. doi: 10.1038/2091095a0. [DOI] [PubMed] [Google Scholar]
- 31.East J, Branca M. Autoimmune reactions and malignant changes in germ-free New Zealand Black mice. Clin Exp Immunol. 1969;4:621–35. [PMC free article] [PubMed] [Google Scholar]
- 32.East J, Prosser PR, Holborow EJ, Jaquet H. Autoimmune reactions and virus-like particles in germ-free NZB mice. Lancet. 1967;1:755–7. doi: 10.1016/s0140-6736(67)91368-2. [DOI] [PubMed] [Google Scholar]
- 33.Maldonado MA, Kakkanaiah V, MacDonald GC, et al. The role of environmental antigens in the spontaneous development of autoimmunity in MRL-lpr mice. J Immunol. 1999;162:6322–30. [PubMed] [Google Scholar]
- 34.Mizutani A, Shaheen VM, Yoshida H, et al. Pristane-induced autoimmunity in germ-free mice. Clin Immunol. 2005;114:110–8. doi: 10.1016/j.clim.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 35.Unni KK, Holley KE, McDuffie FC, Titus JL. Comparative study of NZB mice under germfree and conventional conditions. J Rheumatol. 1975;2:36–44. [PubMed] [Google Scholar]
- 36.Santiago-Raber ML, Baudino L, Izui S. Emerging roles of TLR7 and TLR9 in murine SLE. J Autoimmun. 2009;33:231–8. doi: 10.1016/j.jaut.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 37.Hall JA, Bouladoux N, Sun CM, et al. Commensal DNA limits regulatory T cell conversion and is a natural adjuvant of intestinal immune responses. Immunity. 2008;29:637–49. doi: 10.1016/j.immuni.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fernandes G, Friend P, Yunis EJ, Good RA. Influence of dietary restriction on immunologic function and renal disease in (NZB × NZW) F1 mice. Proc Natl Acad Sci U S A. 1978;75:1500–4. doi: 10.1073/pnas.75.3.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kubo C, Johnson BC, Day NK, Good RA. Effects of calorie restriction on immunologic functions and development of autoimmune disease in NZB mice. Proc Soc Exp Biol Med. 1992;201:192–9. doi: 10.3181/00379727-201-43498. [DOI] [PubMed] [Google Scholar]
- 40.Mizutani H, Engelman RW, Kinjoh K, et al. Calorie restriction prevents the occlusive coronary vascular disease of autoimmune (NZW × BXSB)F1 mice. Proc Natl Acad Sci U S A. 1994;91:4402–6. doi: 10.1073/pnas.91.10.4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hsieh CC, Lin BF. Dietary factors regulate cytokines in murine models of systemic lupus erythematosus. Autoimmun Rev. 2011;11:22–7. doi: 10.1016/j.autrev.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 42.Reifen R, Amital H, Blank M, et al. Linseed oil suppresses the anti-beta-2-glycoprotein-I in experimental antiphospholipid syndrome. J Autoimmun. 2000;15:381–5. doi: 10.1006/jaut.2000.0439. [DOI] [PubMed] [Google Scholar]
- 43.Blank M, Shoenfeld Y. Beta-2-glycoprotein-I, infections, antiphospholipid syndrome and therapeutic considerations. Clin Immunol. 2004;112:190–9. doi: 10.1016/j.clim.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 44.Foxman EF, Iwasaki A. Genome-virome interactions: examining the role of common viral infections in complex disease. Nat Rev Microbiol. 2011;9:254–64. doi: 10.1038/nrmicro2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moon C, Stappenbeck TS. Viral interactions with the host and microbiota in the intestine. Curr Opin Immunol. 2012;24:405–10. doi: 10.1016/j.coi.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lichtman EI, Helfgott SM, Kriegel MA. Emerging therapies for systemic lupus erythematosus--focus on targeting interferon-alpha. Clin Immunol. 2012;143:210–21. doi: 10.1016/j.clim.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.James JA, Harley JB, Scofield RH. Epstein-Barr virus and systemic lupus erythematosus. Current opinion in rheumatology. 2006;18:462–7. doi: 10.1097/01.bor.0000240355.37927.94. [DOI] [PubMed] [Google Scholar]
- 48.Stetson DB, Ko JS, Heidmann T, Medzhitov R. Trex1 prevents cell-intrinsic initiation of autoimmunity. Cell. 2008;134:587–98. doi: 10.1016/j.cell.2008.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reyes A, Haynes M, Hanson N, et al. Viruses in the faecal microbiota of monozygotic twins and their mothers. Nature. 2010;466:334–8. doi: 10.1038/nature09199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Minot S, Sinha R, Chen J, et al. The human gut virome: inter-individual variation and dynamic response to diet. Genome Res. 2011;21:1616–25. doi: 10.1101/gr.122705.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Young GR, Eksmond U, Salcedo R, Alexopoulou L, Stoye JP, Kassiotis G. Resurrection of endogenous retroviruses in antibody-deficient mice. Nature. 2012;491:774–8. doi: 10.1038/nature11599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim YG, Park JH, Reimer T, et al. Viral infection augments Nod1/2 signaling to potentiate lethality associated with secondary bacterial infections. Cell Host Microbe. 2011;9:496–507. doi: 10.1016/j.chom.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zimmermann K, Haas A, Oxenius A. Systemic antibody responses to gut microbes in health and disease. Gut microbes. 2012;3:42–7. doi: 10.4161/gmic.19344. [DOI] [PubMed] [Google Scholar]
- 54.Lathrop SK, Bloom SM, Rao SM, et al. Peripheral education of the immune system by colonic commensal microbiota. Nature. 2011;478:250–4. doi: 10.1038/nature10434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Su LF, Kidd BA, Han A, Kotzin JJ, Davis MM. Virus-Specific CD4(+) Memory-Phenotype T Cells Are Abundant in Unexposed Adults. Immunity. 2013;38:373–83. doi: 10.1016/j.immuni.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]