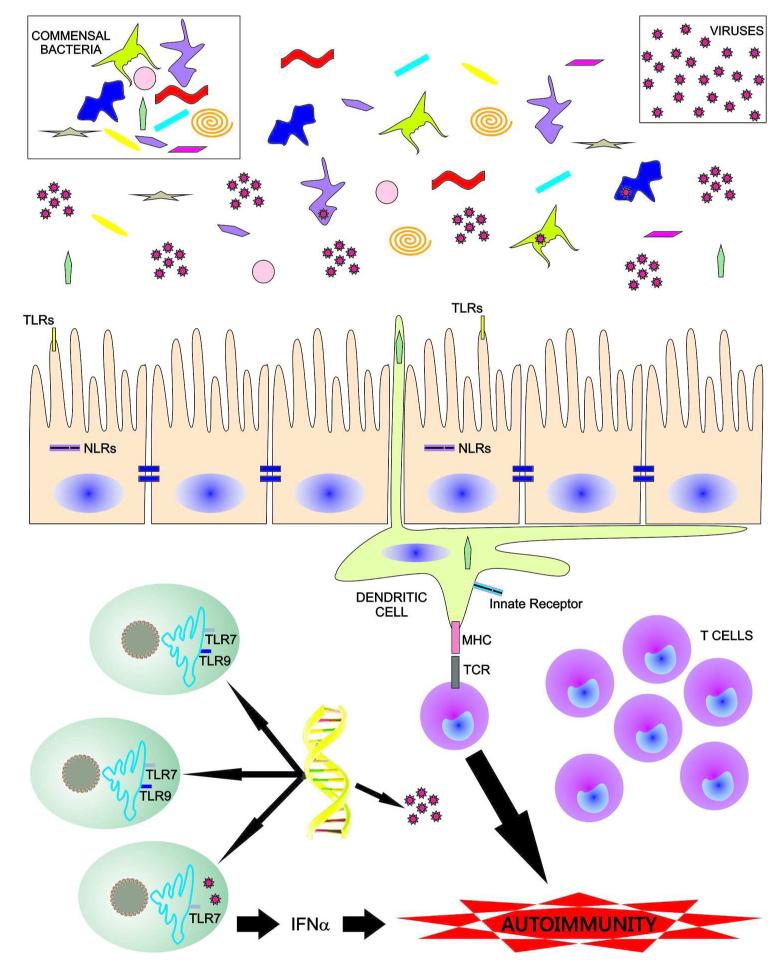
Fig. 1. Putative gut microbiome and virome interactions with an autoimune-prone host immune system.
This cartoon illustrates a hypothetical model for potential mechanisms how the gut microbiota, the gut virome and endogenous retroviruses can contribute to the pathogenesis of systemic autoimmunity. Gut commensals and viruses within the lumen of the gastrointestinal tract are influenced by diet and micronutrients (not shown). Bacteriophages within commensal bacteria are able to modify the bacteria that then affect the host by various mechanisms. Commensals are sensed by specific pattern recognition receptors on the surface or intracellular compartment of epithelial cells and resident antigen-presenting cells, e.g. toll-like receptors (TLRs) or nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs). Dendritic cells can sample commensals within the lumen and present antigens via major histocompatibility complexes (MHC) to T cell subsets that are potentially cross-reactive with self-antigens in a genetically predisposed host. Similarly, genetic predisposition to hypersensitive innate immune receptors or signaling cascades can promote an adaptive immune response to commensal and self antigens. Cross-reactive CD4+ T cells provide subsequently help to inherently autoreactive B cells (not shown) that then produce autoantibodies, leading to systemic autoimmunity. Lastly, endogenous retroviruses that have inserted into the host’s genome (shown in yellow) are also influenced by the gut microbiota and produce nucleic acids that trigger IFNα production via nucleic acid sensors like the endosomally localized TLR7. In addition, commensal DNA triggers a TLR9-mediated response in lamina propria dendritic cells (not depicted). These innate immune events may support an adaptive (auto)immune response to self structures initiated by cross-reactive commensals. See main text for references supporting several of these aspects in non-autoimmune hosts.