Abstract
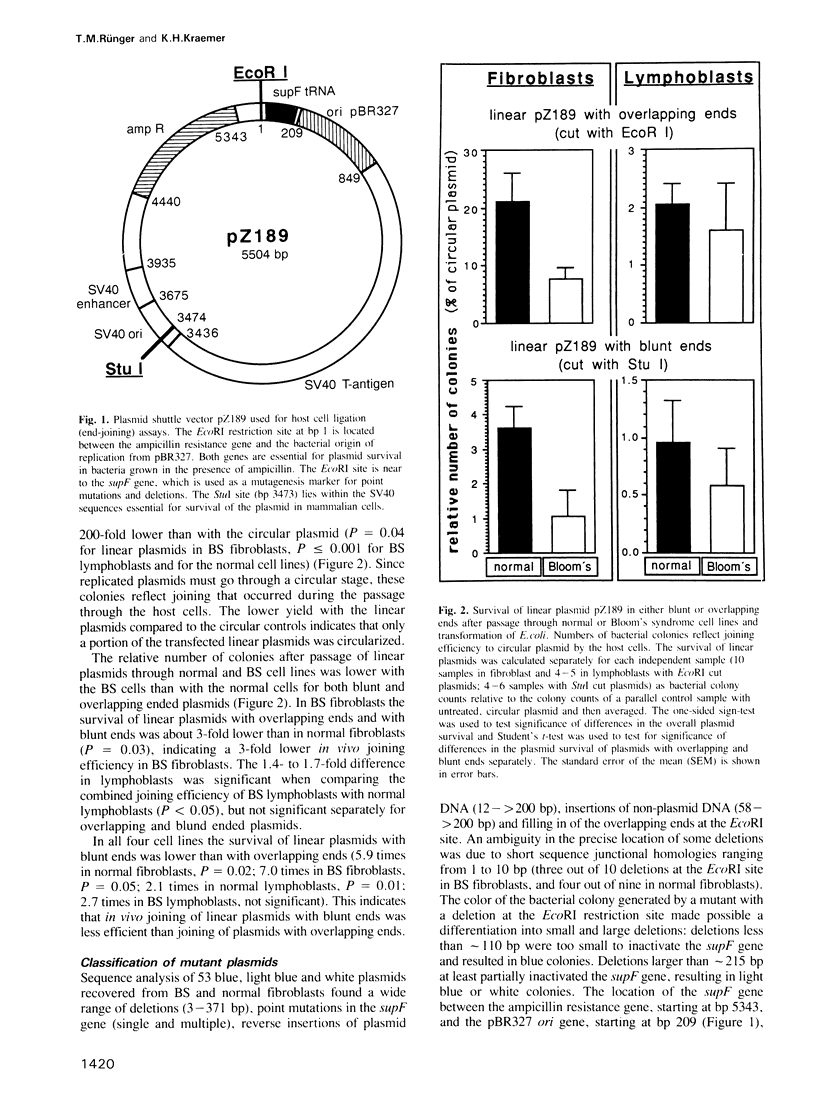
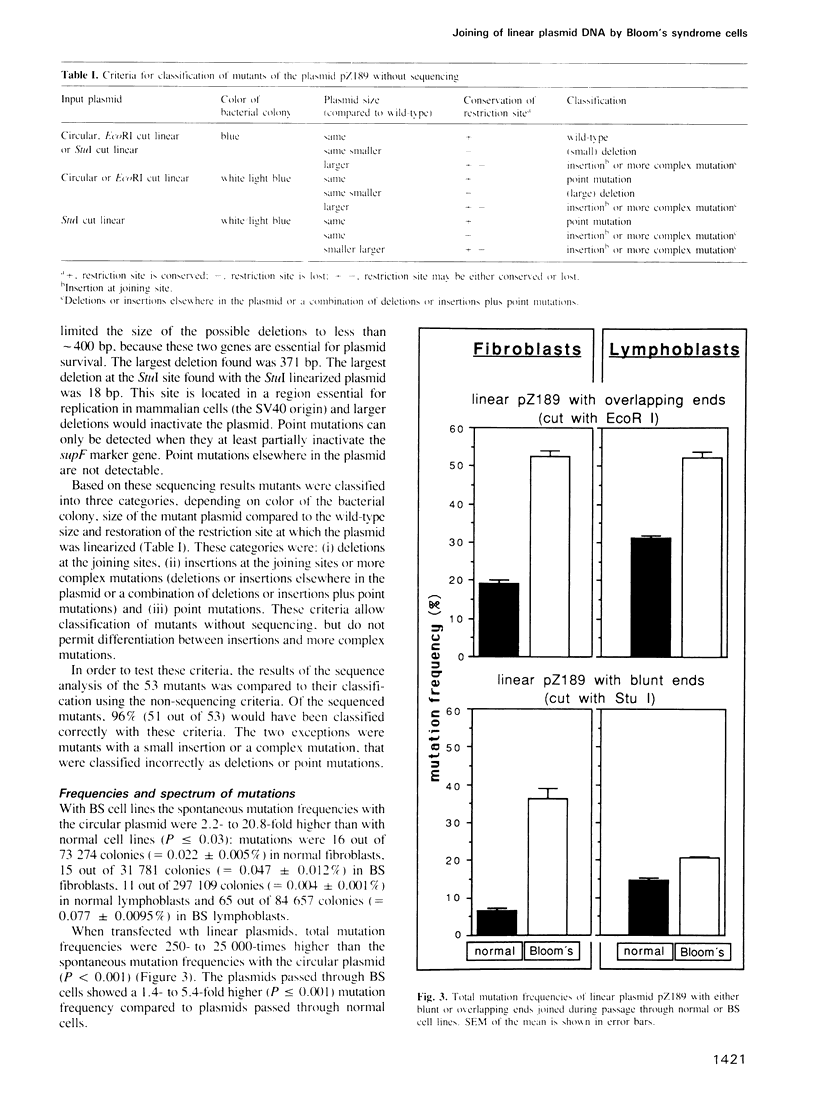
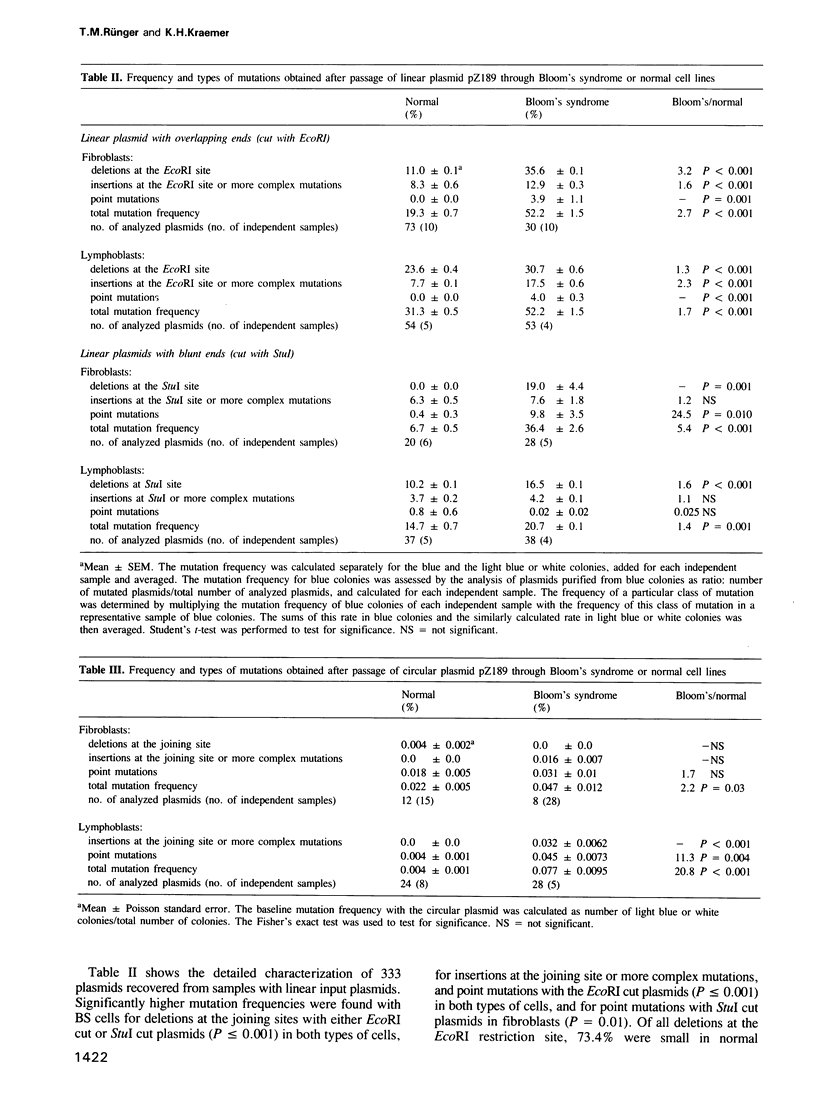
A linearized, replicating, shuttle vector plasmid, pZ189, was used to measure in vivo DNA joining ability of cells from patients with the cancer-prone, immunodeficient, chromosome breakage disorder, Bloom's syndrome (BS). The BS cell lines we studied were reported to contain reduced in vitro activity of DNA ligase I. We assessed in vivo joining ability by transfecting linear plasmids with overlapping or blunt ends (produced by EcoRI or StuI) into BS and normal fibroblast or lymphoblast host cells and measuring the amount of re-joined, replicated plasmids by their ability to transform bacteria. With plasmids having either overlapping or blunt ends we found a 1.3- to 3-fold lower (P less than 0.05) joining efficiency in BS cells than in the normal cells. The mutation frequency of the recovered plasmids was measured by screening for function of the suppressor tRNA contained in pZ189, for plasmid size, for presence of restriction sites, or by DNA sequencing. The spontaneous mutation frequency with the circular plasmid was 0.05-0.08% with both BS cell lines, values 2- to 21-fold higher (P less than 0.03) than with the normal cell lines. The mutation frequency with the linear plasmid passaged through both BS cell lines was 21-52%, values 1.4- to 5.4-fold higher (P less than 0.001) than with the normal lines. Detailed analysis of 210 recovered plasmids revealed an increase (P less than or equal to 0.001) in deletions, insertions or complex mutations at the joining sites, and in point mutations with the EcoRI cut plasmid with the BS cells in comparison to the normal cells.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredberg A., Kraemer K. H., Seidman M. M. Restricted ultraviolet mutational spectrum in a shuttle vector propagated in xeroderma pigmentosum cells. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8273–8277. doi: 10.1073/pnas.83.21.8273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubley G. J., Schnipper L. E. Effects of Bloom's syndrome fibroblasts on genetic recombination and mutagenesis of herpes simplex virus type 1. Somat Cell Mol Genet. 1987 Mar;13(2):111–117. doi: 10.1007/BF01534691. [DOI] [PubMed] [Google Scholar]
- Calos M. P., Lebkowski J. S., Botchan M. R. High mutation frequency in DNA transfected into mammalian cells. Proc Natl Acad Sci U S A. 1983 May;80(10):3015–3019. doi: 10.1073/pnas.80.10.3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German J., Bloom D., Passarge E. Bloom's syndrome XI. Progress report for 1983. Clin Genet. 1984 Feb;25(2):166–174. doi: 10.1111/j.1399-0004.1984.tb00480.x. [DOI] [PubMed] [Google Scholar]
- Gretzula J. C., Hevia O., Weber P. J. Bloom's syndrome. J Am Acad Dermatol. 1987 Sep;17(3):479–488. doi: 10.1016/s0190-9622(87)70233-3. [DOI] [PubMed] [Google Scholar]
- Hesse J. E., Lieber M. R., Gellert M., Mizuuchi K. Extrachromosomal DNA substrates in pre-B cells undergo inversion or deletion at immunoglobulin V-(D)-J joining signals. Cell. 1987 Jun 19;49(6):775–783. doi: 10.1016/0092-8674(87)90615-5. [DOI] [PubMed] [Google Scholar]
- Hütteroth T. H., Litwin S. D., German J. Abnormal immune responses of Bloom's syndrome lymphocytes in vitro. J Clin Invest. 1975 Jul;56(1):1–7. doi: 10.1172/JCI108058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois R. G., Bigbee W. L., Jensen R. H., German J. Evidence for increased in vivo mutation and somatic recombination in Bloom's syndrome. Proc Natl Acad Sci U S A. 1989 Jan;86(2):670–674. doi: 10.1073/pnas.86.2.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber M. R., Hesse J. E., Mizuuchi K., Gellert M. Developmental stage specificity of the lymphoid V(D)J recombination activity. Genes Dev. 1987 Oct;1(8):751–761. doi: 10.1101/gad.1.8.751. [DOI] [PubMed] [Google Scholar]
- Protić-Sabljić M., Kraemer K. H. One pyrimidine dimer inactivates expression of a transfected gene in xeroderma pigmentosum cells. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6622–6626. doi: 10.1073/pnas.82.19.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzaque A., Chakrabarti S., Joffee S., Seidman M. Mutagenesis of a shuttle vector plasmid in mammalian cells. Mol Cell Biol. 1984 Mar;4(3):435–441. doi: 10.1128/mcb.4.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seetharam S., Protić-Sabljić M., Seidman M. M., Kraemer K. H. Abnormal ultraviolet mutagenic spectrum in plasmid DNA replicated in cultured fibroblasts from a patient with the skin cancer-prone disease, xeroderma pigmentosum. J Clin Invest. 1987 Dec;80(6):1613–1617. doi: 10.1172/JCI113248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman M. M., Bredberg A., Seetharam S., Kraemer K. H. Multiple point mutations in a shuttle vector propagated in human cells: evidence for an error-prone DNA polymerase activity. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4944–4948. doi: 10.1073/pnas.84.14.4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman M. M., Dixon K., Razzaque A., Zagursky R. J., Berman M. L. A shuttle vector plasmid for studying carcinogen-induced point mutations in mammalian cells. Gene. 1985;38(1-3):233–237. doi: 10.1016/0378-1119(85)90222-7. [DOI] [PubMed] [Google Scholar]
- Söderhäll S., Lindahl T. DNA ligases of eukaryotes. FEBS Lett. 1976 Aug 1;67(1):1–8. doi: 10.1016/0014-5793(76)80858-7. [DOI] [PubMed] [Google Scholar]
- Taniguchi N., Mukai M., Nagaoki T., Miyawaki T., Moriya N., Takahashi H., Kondo N. Impaired B-cell differentiation and T-cell regulatory function in four patients with Bloom's syndrome. Clin Immunol Immunopathol. 1982 Feb;22(2):247–258. doi: 10.1016/0090-1229(82)90041-1. [DOI] [PubMed] [Google Scholar]
- Ueno Y., Miyawaki T., Seki H., Hara K., Sato T., Taniguchi N., Takahashi H., Kondo N. Impaired natural killer cell activity in Bloom's syndrome could be restored by human recombinant IL-2 in vitro. Clin Immunol Immunopathol. 1985 May;35(2):226–233. doi: 10.1016/0090-1229(85)90068-6. [DOI] [PubMed] [Google Scholar]
- Vijayalaxmi, Evans H. J., Ray J. H., German J. Bloom's syndrome: evidence for an increased mutation frequency in vivo. Science. 1983 Aug 26;221(4613):851–853. doi: 10.1126/science.6879180. [DOI] [PubMed] [Google Scholar]
- Wake C. T., Gudewicz T., Porter T., White A., Wilson J. H. How damaged is the biologically active subpopulation of transfected DNA? Mol Cell Biol. 1984 Mar;4(3):387–398. doi: 10.1128/mcb.4.3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren S. T., Schultz R. A., Chang C. C., Wade M. H., Trosko J. E. Elevated spontaneous mutation rate in Bloom syndrome fibroblasts. Proc Natl Acad Sci U S A. 1981 May;78(5):3133–3137. doi: 10.1073/pnas.78.5.3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weemaes C. M., Bakkeren J. A., ter Haar B. G., Hustinx T. W., van Munster P. J. Immune responses in four patients with Bloom syndrome. Clin Immunol Immunopathol. 1979 Jan;12(1):12–19. doi: 10.1016/0090-1229(79)90107-7. [DOI] [PubMed] [Google Scholar]
- Willis A. E., Weksberg R., Tomlinson S., Lindahl T. Structural alterations of DNA ligase I in Bloom syndrome. Proc Natl Acad Sci U S A. 1987 Nov;84(22):8016–8020. doi: 10.1073/pnas.84.22.8016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. H., Berget P. B., Pipas J. M. Somatic cells efficiently join unrelated DNA segments end-to-end. Mol Cell Biol. 1982 Oct;2(10):1258–1269. doi: 10.1128/mcb.2.10.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodworth-Gutai M. Recombination in SV40-infected cells: viral DNA sequences at sites of circularization of transfecting linear DNA. Virology. 1981 Mar;109(2):353–365. doi: 10.1016/0042-6822(81)90506-7. [DOI] [PubMed] [Google Scholar]


