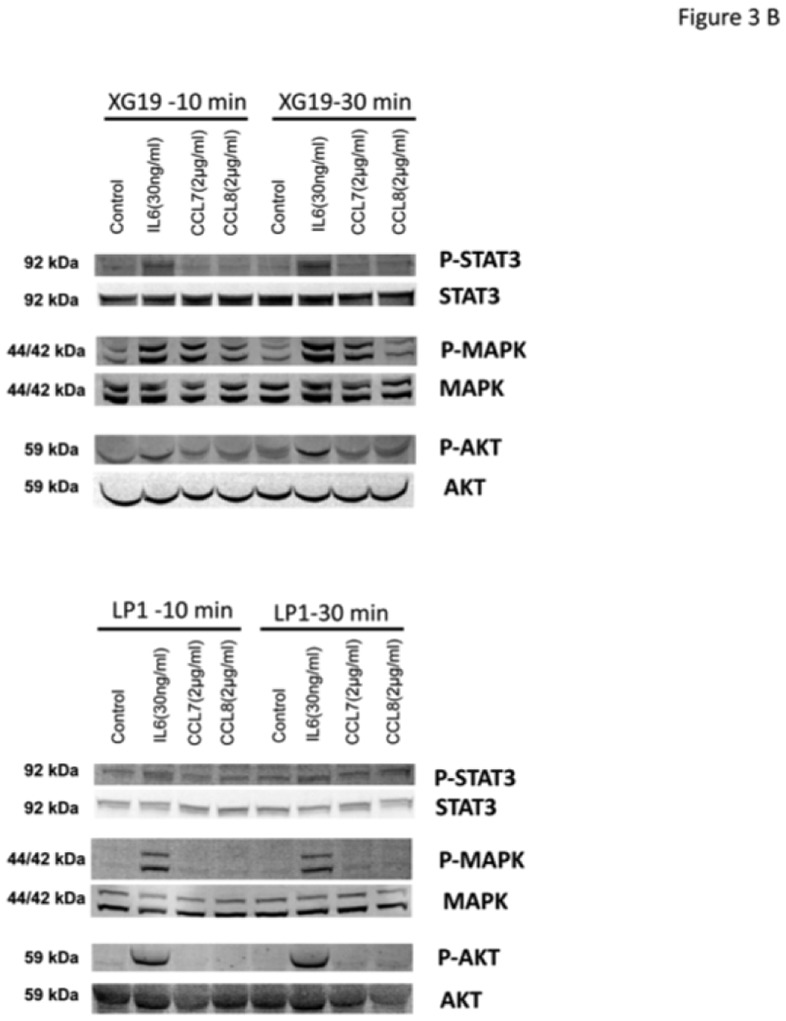
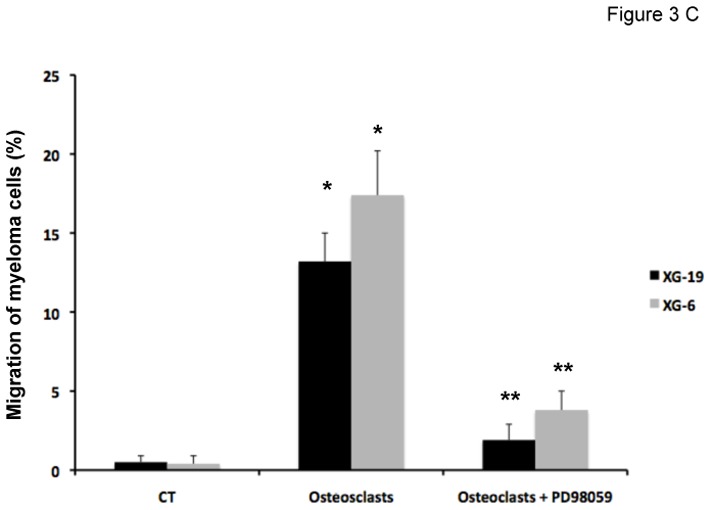
Figure 3. CCL7, CCL8 and CCL13 support the growth of CCR2+ HMCLs.
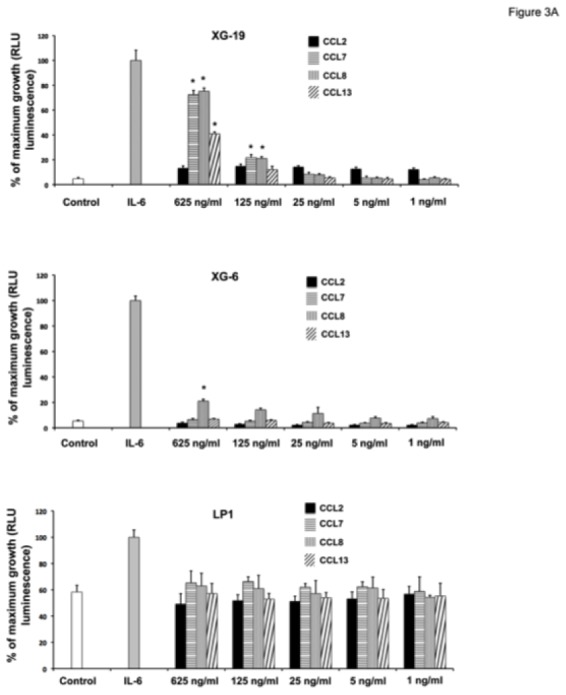
A. XG-19, LP1 and XG-6 were IL-6 starved for 3 hours and cultured either with no cytokine, or in the presence of IL-6 (3 ng/mL) or in the presence of increasing concentrations of CCL2, CCL7, CCL8 or CCL13. Results are the mean ± SD values of the RLU fluorescence determined on sextuplet culture wells. Results are those of one experiment representative of 5. *Mean value is significantly different from that obtained without adding cytokine using a Student t test (P ≤ .05).
B. XG-19 or LP1 cells were starved overnight and cultured without cytokine, or with either IL-6 (30 ng/mL), CCL7 (2 μg/mL), or CCL8 (2 μg/mL) for 10 and 30 minutes at 37°C. Cell lysates were probed by Western blotting with antibodies against phospho-STAT3 (pSTAT3), phospho-ERK1/2 (pMAPK), and phospho-AKT (pAKT). Blots were reprobed with antibodies to STAT3, MAPK, and AKT proteins to quantitate protein loading. Western blots are of one experiment representative of 3.
C. The chemoattractant activity of osteoclasts to XG-19 or XG-6 myeloma cells was assayed with or without a MAPK inhibitor (PD98059). Data are the fraction of primary MMC in the upper chamber of the transwell that could migrate to the lower chamber and are mean values of three experiments. * indicates a significant increase in MMC migration in MMC/osteoclast cocultures compared to MMC alone using a paired Student’s t test (P ≤ .05). ** indicates a significant decrease of MMC migration in MMC/osteoclast cocultures with PD98059 (10 μM) compared to MMC/osteoclast cocultures using a paired Student’s t test (P ≤ .05).
