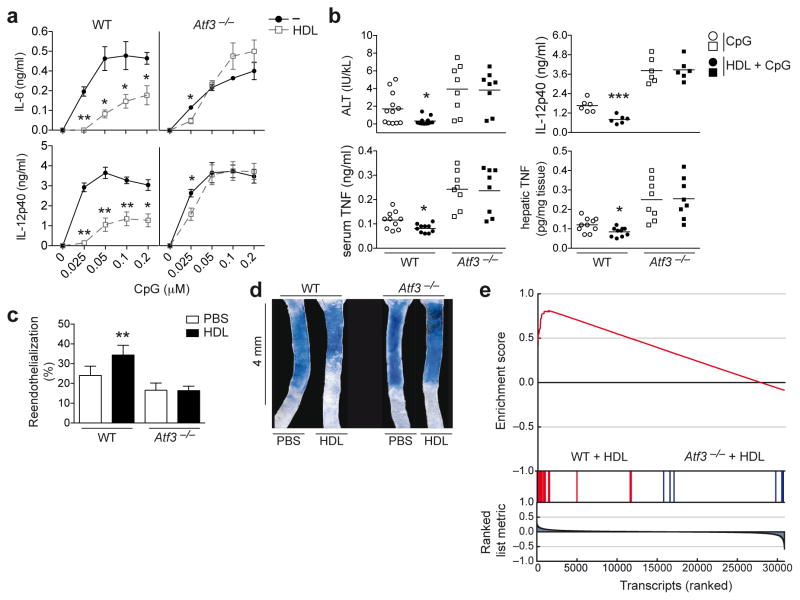
Figure 7.
ATF3 is required for the anti-inflammatory effect of HDL in vitro and in vivo. a, ELISA from WT or Atf3-deficient BMDMs pre-treated with HDL (2 mg/ml) for 6 h prior to overnight stimulation with CpG (indicated doses). b, WT or Atf3-deficient mice injected i.p with HDL (2 mg) 6 h before subsequent injection with CpG (30 μg) and D-gal (10 mg) for a further 10 h. Serum ALT, serum TNF and hepatic TNF (WT n=10, Atf3−/− n=8,), and serum IL-12p40 (n=6) were measured. c,d, HDL increased re-endothelialization after carotid artery injury in WT, but not Atf3-deficient mice. Carotid injury was performed on WT or Atf3-deficient mice, 3 h later PBS or HDL (20 mg/kg) was injected i.v. Re-endothelialization was evaluated 3 days following carotid injury (WT PBS n=8, WT HDL n=7, Atf3−/− PBS n=7, Atf3−/− HDL n=9). e Gene set enrichment analysis using the macrophage/carotid injury overlapping gene set applied to the carotid injury dataset assessing gene enrichment in HDL treated samples derived from WT versus Atf3−/− mice. a, Combined data from three independent experiments are shown as the mean ±S.E.M (WT versus Atf3−/− *p<0.05, **p<0.01). b, Data are presented as mean values ±S.E.M, CpG versus HDL+CpG (per genotype) *p<0.05, ***p<0.001. c, Data are presented as mean values ±S.E.M, PBS vs HDL (per genotype) **p<0.01. d, Images are representative of each group in each genotype. e, At least three biological replicates per condition were generated.