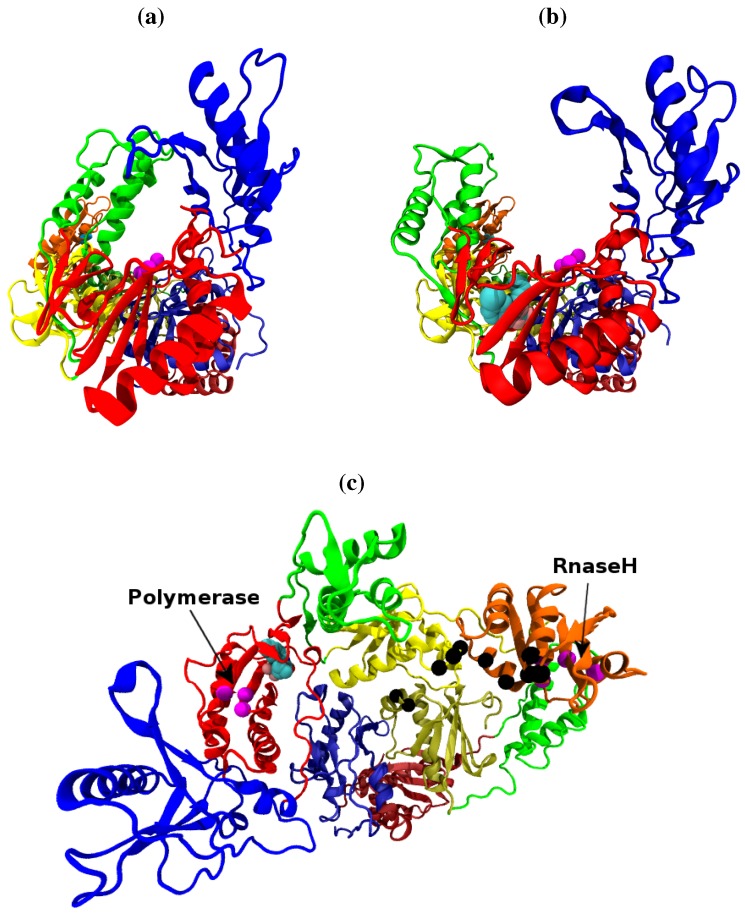
Figure 1.
The structure of the HIV-1 RT shown along the template/primer binding cleft in the (a) closed apo (based on the 1DLO crystal structure) and (b) open NNRTI bound conformation (based on the EFV bound 1IKW structure, the van der Waals surface of the drug is shown). In this view the p66 subunit is most prominent, in (c) viewed from above the template/primer binding cleft both the p66 and p51 monomers are clearly visible. The subunits are named after the structures supposed likeness to a right hand. It is in fact, the folding of the p66 seen in (a) and (b) which results in the likeness. However the subdomains retain their name in p51 as seen in (b). The subdomains are known as the (F)ingers (blue), (P)alm (red), (T)humb (green), (C)onnection (yellow) and (R)NaseH (orange). The first 4 subdomains form the polymerase domain and are common to both subunits. In all figures the residues of the polymerase and RNaseH active sites are shown as magenta beads (the former is located in the palm). The residues of the RNaseH primer grip are indicated by black beads.