Table 1.
Initial Screen with Amines and VANOL, VAPOL and BINOL ligands.a
| |||||||
|---|---|---|---|---|---|---|---|
| entry | ligand | amine | phenol/alcoholb | catalyst# | time (h) | % yield 3c | erd |
| 1 | L-4 | A-1 | P-11 | LAP 4-1-11 | 18 | nde | – |
| 2 | L-4 | A-2 | P-11 | LAP 4-2-11 | 24 | nd | – |
| 3 | L-4 | A-3 | P-11 | LAP 4-3-11 | 24 | nd | – |
| 4 | L-4 | A-4 | P-11 | LAP 4-4-11 | 24 | nd | – |
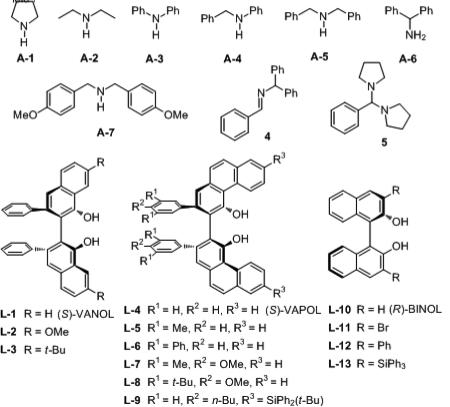
| 5 | L-4 | A-5 | P-11 | LAP 4-5-11 | 24 | 76 | 41 : 59f |
| 6 | L-4 | A-6 | P-11 | LAP 4-6-11 | 18 | ndg | – |
| 7 | L-4 | A-7 | P-11 | LAP 4-7-11 | 48 | 82 | 57 : 43 |
| 8 | L-1 | A-5 | P-11 | LAP 1-5-11 | 36 | 60 | 47 : 53f |
| 9 | L-10 | A-5 | P-11 | LAP 10-5-11 | 40 | 9h | 44 : 56f |
| 10 | L-11 | A-5 | P-11 | LAP 11-5-11 | 24 | 37 | 55 : 45 |
| 11 | L-12 | A-5 | P-11 | LAP 12-5-11 | 43 | 30 | 55 : 45 |
| 12 | L-13 | A-5 | P-11 | LAP 13-5-11 | 24 | trace | – |
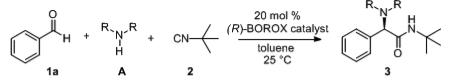
Unless otherwise specified, all reactions were carried out at 0.2 M in 1a (0.25 mmol) in toluene with 2.0 equiv amine and 1.5 equiv of 2 at rt for the indicated time with 20 mol % of the catalyst. The pre-catalyst was prepared by heating 20 mol% of the (R)-ligand, 40 mol% of the phenol or alcohol, 60 mol% H2O, 60 mol% of BH3·SMe2 in toluene at 100 °C for 1 h. After removal of all volatiles, the BOROX catalyst was generated in-situ by the addition of the amine at rt and this was followed by the addition of aldehyde and then the isonitrile.
P-11 is phenol (Table 2).
Isolated yield after chromatography on silica gel. nd = not detected.
Determined by HPLC.
The aminal 5 was formed in 50% yield (1H NMR) and amide 3 could not be detected. Heating this mixture at 80 °C for 18 h resulted in a complex mixture with 3 still not detectable.
Catalyst was generated from (S)-ligand.
The imine 4 was formed in 100% yield as determined by 1H NMR yield with an internal standard. After 89 h, a 100% yield of imine was still observed.
1H NMR yield with internal standard.