Table 2.
Synergism in the Arrangement of the Substituents in the Boroxinate Core.a
| ||||||
|---|---|---|---|---|---|---|
| entry | ligand | phenol/alcohol | catalyst | time (h) | % yield 3b | erc |
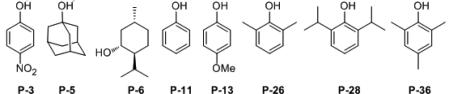
| 1 | L-4 | P-3 | LAP 4-5-3 | 36 | 64 | 57 : 43 |
| 2 | L-4 | P-5 | LAP 4-5-5 | 39 | 86 | 58 : 42 |
| 3 | L-4 | P-6 | LAP 4-5-6 | 24 | 91 | 58 : 42 |
| 4 | L-4 | P-11 | LAP 4-5-11 | 24 | 76 | 59 : 41 |
| 5 | L-4 | P-13 | LAP 4-5-13 | 36 | 75 | 59 : 41 |
| 6 | L-4 | P-26 | LAP 4-5-26 | 39 | 82 | 64 : 36 |
| 7 | L-4 | P-28 | LAP 4-5-28 | 39 | 71 | 60 : 40 |
| 8 | L-4 | P-36 | LAP 4-5-36 | 36 | 72 | 70 : 30 |
| 9 | L-1 | P-36 | LAP 1-5-36 | 39 | 52 | 49 : 51d |
| 10 | L-2 | P-36 | LAP 2-5-36 | 42 | 62 | 54 : 46 |
| 11 | L-3 | P-36 | LAP 3-5-36 | 45 | 62 | 52 : 48 |
| 12 | L-5 | P-36 | LAP 5-5-36 | 39 | 89 | 74 : 26 |
| 13 | L-6 | P-36 | LAP 6-5-36 | 39 | 64 | 35 : 65d |
| 14 | L-7 | P-36 | LAP 7-5-36 | 39 | 93 | 74 : 26 |
| 15 | L-8 | P-36 | LAP 8-5-36 | 39 | 94 | 15 : 85d |
| 16 | L-9 | P-36 | LAP 9-5-36 | 42 | 90 | 71 : 29 |
| 17 | L-8 | P-11 | LAP 8-5-11 | 39 | 74 | 62 : 38e |
| 18 | L-8 | P-26 | LAP 8-5-26 | 39 | 93 | 78 : 22e |
Unless otherwise specified, all reactions were carried out at 0.2 M in 1 (0.25 mmol) in toluene with 2.0 equiv amine and 1.5 equiv of 2 at rt for the indicated time with 20 mol% of the catalyst. The catalyst was made from the (S)-enantiomer of the ligand (≥99% ee) according to the procedure in Table 1.
Isolated yield after chromatography on silica gel.
Determined by HPLC.
Catalyst generated from (R)-ligand.
The ligand for this run was 97% ee.
f The catalyst loading was 10 mol%.