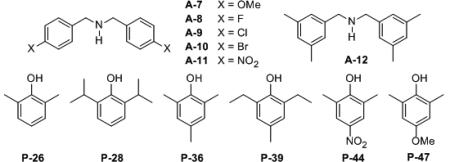
Table 3.
Screen of Additional Phenol Substituents in the Boroxainte Core with Ligand L-8.a
| |||||||
|---|---|---|---|---|---|---|---|
| entry | ligand | amine | phenol/alcohol | catalyst | time (h) | % yield 3ab | erc |
| 1 | L-4 | A-5 | P-11 | LAP 4-5-11 | 38 | 71 | 61 : 39 |
| 2 | L-4 | A-5 | P-36 | LAP 4-5-36 | 42 | 90 | 71 : 29 |
| 3 | L-8 | A-5 | P-11 | LAP 8-5-11 | 41 | 72 | 33 : 67d |
| 4 | L-8 | A-5 | P-26 | LAP 8-5-26 | 39 | 94 | 83 : 17 |
| 5 | L-8 | A-5 | P-28 | LAP 8-5-28 | 39 | 72 | 68 : 32 |
| 6 | L-8 | A-5 | P-36 | LAP 8-5-36 | 39 | 92 | 87 : 13 |
| 7 | L-8 | A-5 | P-36 | LAP 8-5-36 | 39 | 86 | 86 : 14e |
| 8 | L-8 | A-5 | P-36 | LAP 8-5-36 | 39 | 75 | 84 : 16f |
| 9 | L-8 | A-12 | P-36 | LAP 8-12-36 | 39 | 65 | 61 : 39 |
| 10 | L-6 | A-5 | P-36 | LAP 8-5-36 | 41 | 53 | 32 : 68d |
| 11 | L-8 | A-5 | P-39 | LAP 8-5-39 | 38 | 95 | 82 : 18 |
| 12 | L-8 | A-5 | P-44 | LAP 8-5-44 | 38 | 76 | 59 : 41 |
| 13 | L-8 | A-5 | P-47 | LAP 8-5-47 | 38 | 91 | 10 : 90d,g,h |
| 14 | L-8 | A-7 | P-47 | LAP 8-5-47 | 24 | 91 | 12 : 88d |
| 15 | L-8 | A-8 | P-47 | LAP 8-5-47 | 24 | 85 | 10 : 90d |
| 16 | L-8 | A-9 | P-47 | LAP 8-5-47 | 24 | 80 | 12 : 88d |
| 17 | L-8 | A-10 | P-47 | LAP 8-5-47 | 24 | 79 | 13 : 87d |
| 18 | L-8 | A-11 | P-47 | LAP 8-5-47 | 24 | 22 | 27: 73d |
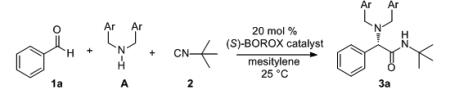
Unless otherwise specified, all reactions were carried out at 0.2 M in 1 (0.25 mmol) in mesitylene with 2.0 equiv amine and 1.5 equiv of 2 at rt for the indicated time with 20 mol% of the catalyst. Entries 14-18 were carried out in the presence of 4Å molecular sieves. The catalyst was prepared according to the procedure in Table 1 with the (S)-ligand unless otherwise specified. Ligand (S)-L-8 was 97% ee and (R)-L-8, (S)-L-4 and (R)-L-6 were >99% ee.
Isolated yield after chromatography on silica gel.
Determined by HPLC.
Catalyst generated from (R)-ligand.
Reaction with 1.2 equiv amine.
Reaction with 1.02 equiv amine.
The er was 89:11 when (S)-L-8 was 97% ee.
The yield was 86% after 24 h.