Table 4.
Substrate Scope of the Catalytic Asymmetric 3-Component Ugi Reaction.a
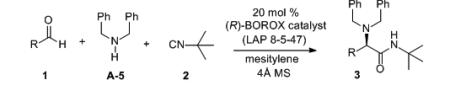
| ||||||
|---|---|---|---|---|---|---|
| entry | series | R | time (h) | temp (°C) | % yield 3b | erc |
| 1 | a | C6H5 | 7 | 40 | 87e | 86 : 14 |
| 2d | a | C6H5 | 24 | 25 | 86 (71) | 90 : 10 (>99.5 : 0.5) |
| 3 | a | C6H5 | 66 | 0 | 75 | 92 : 8 |
| 4f | b | 4-NO2C6H4 | 24 | 25 | 83 | 93 : 7 |
| 5f | b | 4-NO2C6H4 | 66 | 0 | 51e | 92 : 8 |
| 6 | c | 4-CF3C6H4 | 24 | 25 | 85 | 91 : 9 |
| 7 | d | 4-BrC6H4 | 24 | 25 | 85 | 93 : 7 |
| 8 | d | 4-BrC6H4 | 48 | 0 | 65g | 95 : 5 |
| 9f | d | 4-BrC6H4 | 48 | 0 | 75h,i | 95 : 5 |
| 10f | e | 3-BrC6H4 | 22 | 25 | 82 | 93 : 7 |
| 11 | e | 3-BrC6H4 | 66 | 0 | 66h | 92 : 8 |
| 12 | f | 3,4-Cl2C6H3 | 24 | 25 | 85 | 94 : 6 |
| 13 | f | 3,4-Cl2C6H3 | 66 | 0 | 54e | 95 : 5 |
| 14 | g | 4-FC6H4 | 24 | 25 | 87e | 91 : 9 |
| 15 | g | 4-FC6H4 | 67 | 0 | 62 | 94 : 6 |
| 16 | h | 4-MeO2CC6H4 | 24 | 25 | 80 | 93 : 7 |
| 17f | h | 4-MeO2CC6H4 | 67 | 0 | 62 | 93 : 7 |
| 18 | i | 4-OAcC6H4 | 24 | 25 | 86 | 85 : 15 |
| 19 | j | 4-AcNHC6H4 | 24 | 25 | 77 (47) | 85 : 15 (96:4) |
| 20f | k | 4-MeC6H4 | 24 | 25 | 84 (47) | 91 : 9 (>99.5 : 0.5) |
| 21 | k | 4-MeC6H4 | 66 | 0 | 80e | 92 : 8 |
| 22 | l | 2-MeC6H4 | 24 | 25 | 76 (56) | 78 : 22 (>99 : 1) |
| 23 | m | 4-t-BuC6H4 | 24 | 25 | 83 | 84 : 16 |
| 24 | n | 4-MeOC6H4 | 40 | 25 | 84j | 88 : 12 |
| 25 | n | 4-MeOC6H4 | 24 | 25 | 70 | 89 : 11 |
| 28 | n | 4-MeOC6H4 | 24 | 0 | 51 | 92 : 8 |
| 27 | o | 3-pyridyl | 25 | 25 | 80 (61) | 90 : 10 (>99 : 1) |
| 28 | p | 4-pyridyl | 70 | 25 | 66 | 89 : 11 |
Unless otherwise specified, all reactions were carried out at 0.2 M in 1 (0.25 mmol) in mesitylene with 2.0 equiv amine and 1.5 equiv of 2 at rt in the presence of 4Å MS for the indicated time with 20 mol% of the catalyst. The catalyst was made from the (R)-enantiomer of the ligand (≥99% ee) according to the procedure in Table 1.
Isolated yield after chromatography on silica gel. Yield in paranthesis is % recovery of the first crop after crystallization
Determined by HPLC. The er in parentheses is of the first crop.
Average of 4 runs.
1H NMR yield with internal standard (Ph3CH).
Average of 2 runs.
Yield was 46% after 24 h.
Reaction at 0.4 M.
Yield was 76% after 66 h. The yield was 60% after 66 h in the absence of 4Å MS.
Run in the absence of 4Å MS.
