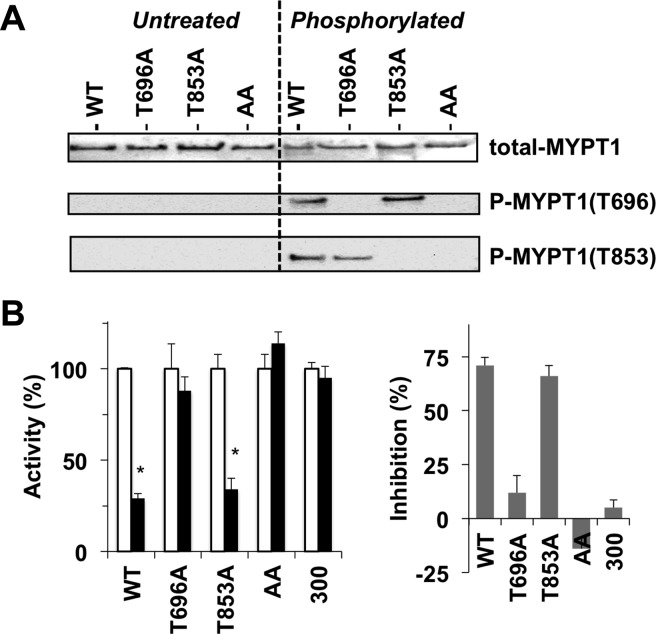
Figure 2.
Phosphorylation-dependent inhibition of the recombinant MLCP complex. (A) Phosphorylation of recombinant MLCP. Untreated (left) or phosphorylated recombinant MLCP with MYPT1 wild type (WT) and T696A, T853A, and T696A/T853A (AA) mutants by ROCK (100 milliunits) (right) was subjected to immunoblotting using antibodies for total-MYPT1, phospho-Thr696, and phospho-Thr853. No phosphorylation was detected with untreated MLCP. (B) MLCP assay. Each MLCP preparation was thio-phosphorylated for 90 min at 30 °C with ROCK (100 milliunits) and 0.1 mM ATPγS prior to the MLCP assay. The relative activity of thio-phosphorylated MLCP (filled bar) was normalized against the untreated enzyme (empty bar) (left). The mean value ± SEM was obtained from triplicate assays with at least three independent thio-phosphorylated MLCP trials. An asterisk indicates p < 0.05 compared to the value with the untreated enzyme. The difference in activity between thio-phosphorylated and untreated enzymes was defined as inhibition (%) (right).