Abstract
Currently, no reliable noninvasive methods exist for monitoring the severity of in vivo cyanide (CN) toxicity, treatment, and resulting physiological changes. We developed a broadband diffuse optical spectroscopy (DOS) system to measure bulk tissue absorption and scattering. DOS was ased to optically monitor CN toxicity and treatment with sodium nitrite (NaNO2). To perform experiments, the DOS probe was placed on the hind leg of rabbits. A sodium CN solution was infused intravenously. DOS and concurrent physiologic measurements were obtained. After completion of CN infusion, NaNO2 was infused to induce methemoglobinemia (MetHb). During infusion of CN, blood gas measurements showed an increase in venous partial pressure of oxygen (pO2), and following reversal, venous pO2 values decreased. DOS measurements demonstrated corresponding changes in hemoglobin oxygenation states and redox states of cytochrome-c oxidase (CcO) during CN infusion and NaNO2 treatment. Therefore, DOS enables detection and monitoring of CN toxicity and treatment with NaNO2.
INTRODUCTION
Cyanide (CN)-based derivatives have been used for centuries as poisons and chemical weapons.1,2 As a highly toxic and volatile substance, CN continues to pose a major potential chemical threat to civilians and military personnel.2,3 There are numerous sources of chemical CN exposure including military and industrial. More commonly, exposure to cyanide occurs during house fires because of the combustion of plastics such as acrylics and acrylonitriles that together with carbon monoxide, aldehydes, and soot further worsen tissue hypoxia, resulting in combustion-related fatalities.4–10 Studies of residential fires have found higher blood CN concentrations in deceased victims than in the survivors, suggesting CN toxicity may be a major component of morbidity and mortality in addition to carbon monoxide poisoning, and combined exposure appears to increase the toxicity of both.4–10
Currently, there is no rapid method to measure cyanide levels in the body. Urine and whole blood analysis are the only regularly used diagnostic tests for cyanide. These methods both require time for collection and interpretation of results.6 Accurate determination of blood CN levels are affected by both the time between exposure and specimen collection and the conditions of blood storage.11–13 The cyanide molecule becomes unstable when exposed to temperatures higher than 4°C; therefore, the samples must be handled properly and processed expeditiously to ensure the accuracy of results.14 Thus, the need for rapid identification of patients exposed to CN and the ability to continuously monitor response to treatment in a field or hospital setting is vital.
The mechanisms of CN toxicity are complex.15,16 The main action of cyanide is an impairment of the ability of tissues to utilize oxygen through inhibition of cytochrome-c oxidase, which ultimately blocks adenosine triphosphate (ATP) synthesis.3 Cytochrome-c oxidase is the terminal oxidase of the mitochondrial respiratory chain and transfers electrons from ferrocytochrome c to molecular oxygen.17 CN has a high binding affinity for active sites on cytochrome-c oxidase.2,3,17–20 When bound, the electron transport chain is arrested with the near infrared (NIR) optically active copper core in reduced form, preventing the donation of an electron.21–25 Progressive cytotoxic tissue hypoxia develops quickly and immediate intervention is necessary to prevent toxicity or death.18 Thus, the need for rapid identification is compounded by the potentially large number of people with significant risks of severe injury from intentional or accidental mass casualty exposure events.
Until recently, CN toxicity treatment in the United States traditionally involved the administration of nitrites to induce methemoglobinemia (MetHb) followed by delivery of thiosulfates to promote excretion.3,26–28 This is administered by holding gauze saturated with amyl nitrite under a patient's nose until intravenous access is obtained. Once an intravenous line has been established, amyl nitrite is discontinued and sodium nitrite is administered. This oxidizes the ferrous ions of hemoglobin to ferric ion. The result is methemoglobin, which strongly binds to cyanide as cyanomethemoglobin. After this is completed, sodium thiosulfate is administered to promote the enzymatic conversion of CN to thiocyanate, which is readily processed by the body and excreted in urine29,30 This treatment method carries considerable risks including suboptimal nitrite dosing, or alternatively, overdosage leading to excessive MetHb, decreased oxygen carrying capacity because of MetHb formation (particularly when combined with carbon monoxide toxicity), profound vasodilation and hypotension, and methylene blue-related complications.26,31 Although a newer, less toxic treatment using hydroxocobalamin has been recently approved by the Food and Drug Administration,32 the limited shelf life, risk of anaphylaxis, requirement for high-volume intravenous administration, and extremely high costs of replacing all cyanide treatment kits throughout the U.S. has slowed widespread use. Although hydroxocobalamin has a high binding affinity for CN, and once bound, forms the nontoxic cyanocobalamin (Vitamin B12) MetHb induction will still be widely available for future CN toxicity events.2,3,33–35 Near infrared diffuse optical spectroscopy (DOS) is a noninvasive optical technology with the ability to simultaneously determine tissue scattering and absorption properties and has the potential to provide capabilities for CN toxicity treatment monitoring by assessing the pathophysiologic changes associated with cyanide toxicity and its reversal.36–42 These changes include an increase in oxyhemoglobin, decrease in deoxyhemoglobin, and an increase in the reduced form of cytochrome-c oxidase during the induction of cyanide toxicity. Conversely, when cyanide toxicity is reversed with sodium nitrite. DOS has the ability to follow the decrease of oxyhemoglobin, increase of deoxyhemoglobin, the cytochrome-c oxidase oxidation, and the increase of methemoglobin in the tissue. The ability of DOS to simultaneously measure tissue scattering and absorption enables accurate, quantitative determination of the concentration of NIR-absorbing solutes in bulk tissues in vivo.43–45 We propose that DOS, which can noninvasively quantify tissue oxy-, met-, and deoxy hemoglobin,38–41 methylene blue levels,42 as well as changes in cytochrome-c oxidase redox states, would be a potentially ideal technology to fulfill the need for cyanide toxicity treatment monitoring.
We have previously shown that DOS can be used to detect the physiologic events occurring during development of CN toxicity in an animal model.46 Therefore, in this study, we extend the investigations to determine the feasibility of using a noninvasive DOS prototype device we designed and constructed to detect the physiologic and biochemical events during sodium nitrite-induced methemoglobin-based treatment of CN toxicity in a rabbit model.
MATERIAL AND METHODS
General Preparation
The procedures involving live vertebrate animals were reviewed and approved by the fully accredited University of California Irvine (UCI) Institutional Animal Care and Use Committee (IACUC). Male New Zealand white rabbits (N = 6) (Myrtle's Rabbitry Inc., Thompson Station, Tennessee) weighing 4.0 ± 0.5 kg were anesthetized with an intramuscular (IM) combination of Ketamine HCl 50 mg/kg (Ketaject, Phoenix Pharmaceutical Inc., St. Joseph, Michigan) and Xylazine 5 mg/kg (Anased, Lloyed Laboratories, Shenandoa, Iowa), using a 23-gauge 5/8-inch needle. After the IM injection, a 23-gauge, 1-inch catheter was placed in the animal's marginal ear vein to administer intravenous (IV) anesthesia. A continuous infusion of 0.7 mg/kg/min Ketamine and 0.14 mg/kg/min Xylazine was used as maintenance anesthetic throughout the procedure. A plane 2 (loss of blink reflexes, fixed pupils, regular respiration with ventilator assistance) depth of anesthesia was maintained throughout the surgery, by monitoring the physical reflexes of the animal.
The animals were intubated with a 3.0-cuffed endotracheal tube, which was secured by a gauze tie, and mechanically ventilated (dual phase control respirator, model 32A4BEPM-5R. Harvard Apparatus, Chicago, Illinois) at a respiration rate of 32/min and a tidal volume of 50 mL and a fraction of inspired oxygen (FiO2) of 100%. A pulse oximeter (Biox 3700 Pulse Oximeter, Ohmeda, Boulder, Colorado) with a probe placed on the tongue was used to measure saturation of hemoglobin with oxygen (SPO2). Upon completion of the experiment, the animals were euthanized with an intravenous injection of Eutha-6 (65 mg/kg) administered through the marginal ear vein.
Systemic Arterial Blood Pressure, Blood Gas Analysis, and Complete Blood Counts
Femoral arterial and venous cutdowns were performed for central line placement to collect blood samples and measure systemic pressure. A 3-inch incision was made with a 10-blade scalpel blade on the shaved, left hind leg of the animal, and blunt dissection was used to isolate the vein and artery. A 12-inch (4 French). 18-gauge catheter (C-PMA-400-FA, Cook Inc., Bloomington, Indiana) was inserted into each, and a 3-way stopcock was placed on the ends. Systemic arterial pressure was monitored using a calibrated pressure transducer (TSD104A Transducer and MP100 WSW System, Biopac Systems, Inc., Santa Barbara, California). Blood pressure was monitored to assure the stability of the animal during the procedure. Blood was withdrawn from both the arterial and venous lines and measured by a blood gas analyzer (IRMA SL Series 2000 Blood Analysis System, Diametrics Medical Inc., St. Paul, Minnesota) to obtain ABG and VBG. Additional blood was drawn from the artery and was sent to an outside facility (Antech Diagnostics, Irvine, California) for complete blood count (CBC) analysis and cyanide levels of whole blood.
Noninvasive Measurements Using Diffuse Optical Spectroscopy (DOS)
Diffuse optical spectroscopy measurements were obtained through a fiber-optic probe with a light diode emitter and detector at a fixed distance (1.0 cm) from the source fiber, which was placed in the surface of the shaved right, hind, inner thigh of the animal. The broadband DOS prototype system we constructed38,43–48 combines multifrequency domain photon migration (FDPM) with time-independent near infrared (NIR) spectroscopy to accurately measure bulk tissue absorption and scattering spectra. The DOS prototype employs six laser diodes at discrete wavelengths (661, 681, 783, 823, 850, and 910 nm), and a fiber coupled avalanche photo diode detector for the frequency domain measurements. The reduced scattering coefficients are calculated as a function of wavelength throughout the NIR region by fitting a power law to these six reduced scattering coefficients. The steady-state acquisition is a broadband reflectance measurement from 600 to 1000 nm that follows the frequency domain (FD) measurements using a tungsten-halogen light source (FiberLite lamp) and a miniature spectrometer (Ocean Optics USB2000), The intensity of the steady-state (SS) reflectance measurements are calibrated to the FD values of absorption and scattering to establish the absolute reflectance intensity. Finally, the tissue concentrations of oxyhemoglobin (OHb) deoxyhemoglogin (RHb), MetHb, and H2O are calculated by a linear least squares fit of the wavelength-dependent extinction coefficient spectra of each chromophore. We used OHb, RHb, and MetHb absorption spectra reported by Zijlistra49 for the subsequent fitting and analysis.
CN Infusion and Toxicity Reversal
A sodium CN solution of 8 mg/60 cc normal saline was infused over 40 to 60 minutes. After this was completed, a sodium nitrite solution of 8 mg/mL was infused at a rate of 1.4 cc per minute for another 40 to 60 minutes. Both solutions were infused through the femoral vein using an automated infuser (GENIE Plus Infusion and Withdrawal Syringe Pump, Kent Scientific, Torrington, Connecticut). During this time DOS measurements were taken continuously and venous and arterial blood gases were drawn every 15 min for oxygen content and CN levels. Each set of measurements took an average of 5 min to complete.
RESULTS
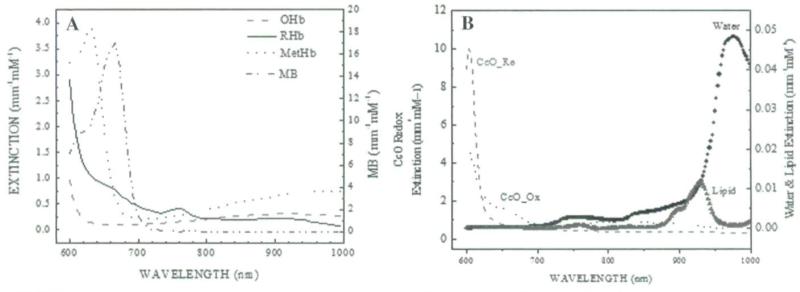
Figure 1 shows the extinction coefficients of the main chromophores in the near infrared wavelength range between 600 and 1000 nm. Figure 1A shows the extinction coefficients of OHb, RHb, and MetHb and Figure 1B shows the extinction coefficients of the redox states of cytochrome-c oxidase, water, and lipid.39,42,50,51 The measurement results from broadband DOS were compared with co-oximetry data from the venous blood samples.
FIGURE 1.
Extinction coefficients of main tissue chromophores in the near infrared wavelength ranges between 600 and 1000 nm. (A) Oxy-, deoxy, and methemoglobin extinction coefficients. (B) The redox states of cytochrome c oxidase, water, and lipid extinction coefficients.
In the following sections, the concentration of chromophores is represented with square brackets. For example, the tissue concentration of oxyhemoglobin is shown as [OHb].
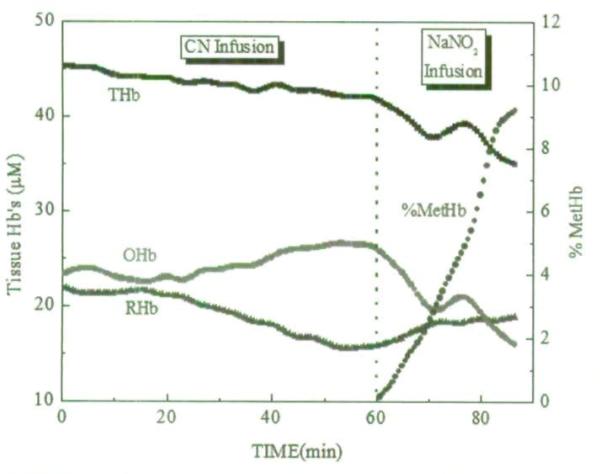
Figure 2 depicts the progression of OHb, RHb, total hemoglobin (THb), and MetHb fraction during CN infusion and after NaNO2 infusion quantified from the broadband DOS measurements. The measurements shown in this figure were acquired continuously every 36 seconds from a single animal. Figure 2 clearly shows the increase in OHb and decrease in RHb during CN infusion and the reversal during NaNO2 infusion. The increase in % MetHb ([MetHb]/([OHb]+[RHb]+[MetHb])*100%) following the start of the NaNO2 infusion started is seen.
FIGURE 2.
Changes in oxyhemoglobin, deoxyhemoglobin, total hemoglobin, and methemoglobin fraction during cyanide infusion and after sodium nitrite infusion quantified from the broadband DOS measurements. The measurements were acquired continuously every 36 seconds from a single animal.
From the venous gas analysis, the partial pressures of the venous blood were 35.1 ± 3.5 mmHg, 46.5 ± 6.2 mmHg, and 24.3 ± 2.9 mmHg at baseline, post CN infusion, and post NaNO2 infusion, respectively. The respective blood cyanide levels were 0.0, 97 ± 28, and 407 ± 107 mg/dL. Both blood analyses are indicative of cyanide toxicity.
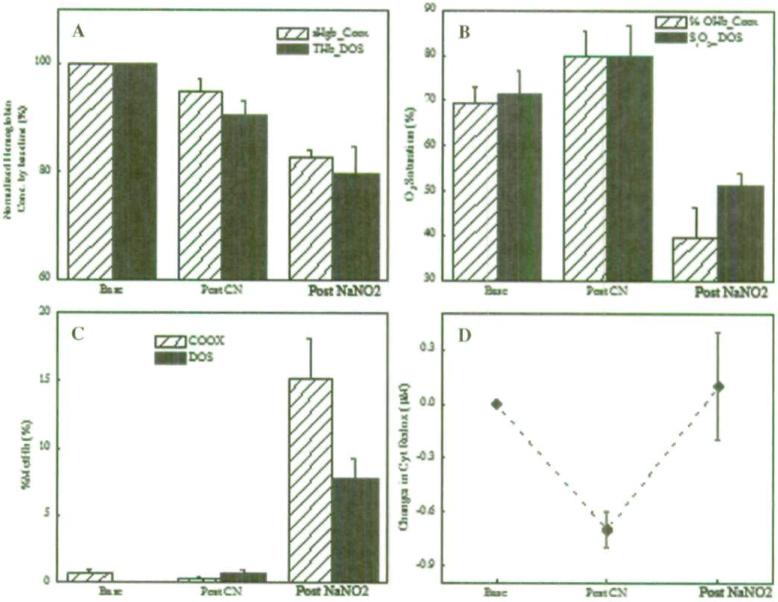
Figure 3 details the comparisons between co-oximetry measurements from venous blood and noninvasive broadband DOS measurements from 6 animals at baseline, post CN infusion, and post NaNO2 treatments. Figure 3A shows hemoglobin concentration (sHgb) by co-oximetry vs. broadband DOS THb normalized by the respective baseline values. Both co-oximetry and broadband DOS THb values decreased throughout the experiment. A similar trend in THb is shown in Figure 2. Figure 3B shows the changes in OHb fraction from the co-oximetry and tissue oxygen saturation (S1O2) from broadband DOS measurements. The co-oximetry values were obtained from venous blood samples, and the results correlated well with pO2 measurements from blood gas analysis. Figure 3C shows the changes in % MetHb from both the co-oximetry and the broadband DOS measurements. Figure 3D shows the changes in redox states of cytochrome-c oxidase at the baseline, immediately post CN infusion and post NaNO2 infusion. The results in Figure 3D indicate that redox states of cytochrome-c oxidase shifted toward a more reduced state during the CN infusion and reversed back toward baseline values after sodium nitrite infusion. Even though a “gold standard” measurement of cytochrome-c oxidase redox states is not available, the results from blood gas analysis, CN level tests, co-oximetry measurements, and broadband DOS measurements are all consistent in indicating the development of cyanide toxicity of animals and the subsequent reversal of toxicity.
FIGURE 3.
Comparison between co-oximetry measurements from the venous blood and noninvasive broadband DOS measurements from 6 animals at the baseline, post cyanide infusion, and post sodium nitrite (NaNO2) treatment. (A) Normalized co-oximetry hemoglobin concentration (sHgb) vs. THb by the baseline values. (B) Co-oximetry oxyhemoglobin fraction vs. broadband DOS tissue oxygen saturation (S1O2). (C) Percent methemoglobin values from co-oximetry and broadband DOS. (D) Changes in redox states of cytochrome c oxidase (ΔCcO_oxidized-ΔCcO_reduced) measured by broadband DOS. All seven variables were found statistically significant (p < 0.05) by repeated measures analysis of variance (ANOVA) tests (SYSTAT version 10, SPSS, Inc.). Bars represent standard error.
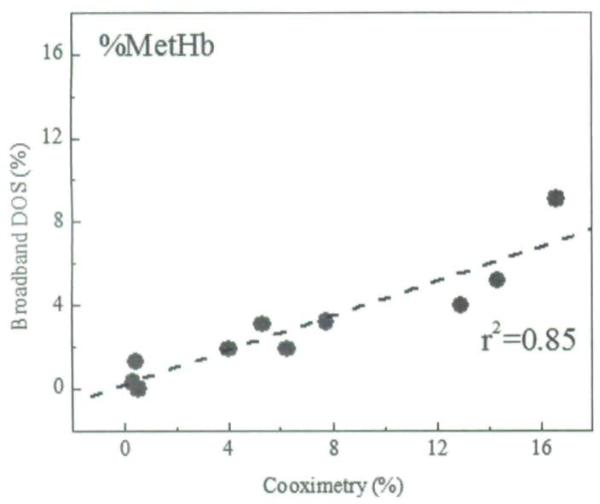
Finally, Figure 4 shows the correlation between co-oximetry and broadband DOS % MetHb values (r2 = 0.85).
FIGURE 4.
Correlation between co-oximetry vs. broadband DOS percent methemoglobin values. r2 denotes Pearson's correlation r2 value.
DISCUSSION
CN toxicity is potentially lethal and can progress very rapidly. Therefore, early and accurate diagnosis as well as determination of the extent of exposure is essential. The only readily available methods to detect CN exposure are blood or urine CN level tests.52 In addition to the previously mentioned limitations of blood cyanide level testing,11,13 MetHb formation may actually increase measured blood CN levels from release of CN from cyanomethemoglobin during the assay process, despite reversing the toxic effects.3,26 In contrast, noninvasive DOS methods are potentially capable of detecting the physiologic manifestations and biochemical effects of CN toxicity and treatment as seen in this study, even though blood levels of CN are not directly measured.
In this study we evaluated the capabilities of DOS for determining the physiological effects resulting from infusion of CN to induce toxicity followed by the infusion of sodium nitrite to reverse its toxic effects. This study was based on our previously described CN model,46 which was designed to study the capabilities of DOS to optically monitor the induction of CN toxicity. The prior study46 showed that DOS was able to measure the severity of in vivo CN toxicity and resulting physiological changes noninvasively. In the current study, we investigated the ability to monitor the CN toxicity treatment reversal process with DOS. To validate the results, DOS measurements were compared to arterial and venous blood gases and also with hemoglobin co-oximetry (the standard method for blood sampling).
The current standard therapies for CN toxicity include either induction of methemoglobinemia or direct binding of CN with hydroxocobalamin. Nitrite-induced MetHb production is used in CN treatment because CN ions have a high affinity for MetHb and Hb is present in very high concentrations in the blood. This high affinity leads to binding of CN in the extracellular space, which results in transfer of CN from cytochrome-c oxidase (though nitrites may also have additional mechanisms of action including direct effects on cytochrome-c oxidase activity).2,3,16,28 The overall effect is the removal of CN from cytochromes and unblocking of the electron transport process. The first step in methemoglobin induction in this treatment involves the inhalation of amyl nitrite vapor followed by intravenous administration of sodium nitrite until the methemoglobin level reaches the desired 10–20% of total hemoglobin.2 A level of MetHb of up to 20–30% total hemoglobin is usually tolerated well by healthy adults.2,26
Although this method of treatment may reduce the severity of CN poisoning, it may also lead to other physiological complications associated with MetHb. Although MetHb molecules have beneficial effects in binding CN, they become incapable of binding and delivering oxygen into tissues. This leads to abnormal oxygen affinity, reduced oxygen-carrying capacity, and potentially to tissue hypoxia.2 As the levels of MetHb approach 30%, onset of physical complications begin to develop including dizziness, fatigue, and shortness of breath.3 Consequently, induction of MetHb may be contraindicated in patients with concurrent carboxyhemoglobinemia (a frequent occurrence with exposure to fires and combustion of CN-forming materials), because of further decreased oxygen carrying capacity of the blood.3
Thus, induction of MetHb for treatment of CN toxicity carries risks, and methods for careful monitoring of methemoglobin induction and effects could be of substantial clinical benefit in CN treatment as well as MetHb toxicity. Furthermore, accurate detection of the appearance of MetHb during nitrite administration could potentially be useful as a guide for continued therapy because MetHb binds cyanide to form cyano-MetHb (which is not detected as MetHb).26 Thus rising MetHb concentrations suggest that little or no free cyanide remains. In this study, we show the ability of DOS to accurately measure MetHb formation and concentration providing a potential method for improving the safety and precision of the MetHb induction process.
CcO is a terminal oxidase in aerobic respiration. This enzyme is involved in >95% of the oxygen consumption in the body and is essential for the efficient generation of cellular ATP.53 Four redox active metal centers are present in CcO: two hemes (Cyt a and Cyt a3) and two coppers (CuA and CuB). Cyt a and CuA mediate electron transfer from cytochrome c to the oxygen reduction site that contains Cyt a3, and CuB. Cyt a3 is a site for binding respiratory inhibitors including CN, carbon dioxide, nitric oxide, nitrate, and oxygen. Their metal centers give rise to absorption bands in the near infrared region.54 Especially in the NIR region between 600 and 1000 nm, the oxidized states have broad absorptions centered at 830 nm, which are associated with the CuA center. As these four metal centers undergo changes in redox states, they give rise to changes in absorption spectra. Since the spectral contribution of CuB associated with Cyt a3 is reported to be low (in the range of 0–15%).21 DOS can be used to follow the redox changes of CcO from absorption band changes because of CuA and Cyt a. As cyanide binds to Cyt a3 of CcO, it prevents oxygen reduction by electrons leaving CuA and Cyt a. As a result, both of these NIR optically predominant metal centers become reduced.22–24 The reduction of these metal centers and binding of CN ligands to CcO leads to the appearance of a strong absorption band at 605 nm23,25 and concomitant absence of absorption bands at 655 nm and 830 nm.55 DOS can detect the changes observed in the near infrared absorption spectra of cytochrome-c oxidase.
Results from our previous studies and this current investigation demonstrate the progressive reduction of cytochrome-c oxidase during CN toxicity observed by DOS. These changes are concurrent with the increase in mixed venous oxygenation measured by co-oximetry and the increases in tissue hemoglobin saturation measured by DOS. During reversal of CN toxicity with the formation of MetHb, the cytochrome-c oxidase reduction is reversed in parallel with a decrease in mixed venous oxygen content and tissue hemoglobin oxygen saturation (as tissues are again able to extract oxygen from the circulating blood) as measured by DOS.
There is a risk of development of toxic levels of MetHb from excessive nitrite administration. MetHb can be reversed with administration of methylene blue, a cofactor for NADPH methemoglobin reductase, an alternative enzyme pathway in methemoglobin reduction. In prior studies, we demonstrated the ability of DOS to measure methylene blue concentrations following intravenous administration.42 Continuous monitoring of tissue hemoglobin oxygen saturation and cytochrome-c oxidation state during MetHb reversal should help to ensure against recurrence of CN toxicity from premature reversal or identify persistent CN toxicity sources.
Given this complexity of events during CN toxicity and treatment, DOS should make it possible to more reliably, safely, and noninvasively monitor the phases of CN toxicity, MetHb formation, and reversal.
There are a number of limitations of the study. The purpose of this investigation was to demonstrate feasibility of using DOS technologies for detection of the physiologic effects of cyanide toxicity and monitoring response to therapy. As such, we did not demonstrate definitive efficacy of MetHb treatment in comparison to control animals, nor in comparison to other forms of treatment. Additionally, CN levels remained high in the treatment animals because we did not seek complete reversal of CN. Because we did not follow MetHb induction with thiosulfate, CN was not rapidly excreted from the body, and therefore remained in the animal bloodstream. All animals in these studies are anesthetized for comfort and safety assurances in compliance with animal welfare regulations. Effects of anesthesia on the physiologic responses to CN poisoning and response to treatment cannot be assessed.
Specific DOS limitations include the fact that DOS measures average tissue constituents to a depth of approximately 2–4 cm (at the source detector separation of 10 mm used here). The current system measures only one site, which is placed over muscle, but includes contributions from skin and fat layers. Regional variability, or specific organ dysfunction that are known to occur with CN exposure2 would not be detected with the current setup. Future designs with collection in parallel from multiple source-detector separations and sites could reduce potential variability from these factors and tissue heterogeneity would be better characterized. In addition, within the DOS hemoglobin signals, there may be some interference of tissue myoglobin or other NIR absorbers not accounted for in the analysis.
DOS appears to provide a novel tool for future studies of CN toxicity and reversal, may be helpful for studies comparing the safety and effectiveness of these various treatment regimens, and has potential to be useful for a range of clinical conditions where in-vivo NIR-absorbing chromophore concentration measurements may be beneficial.
ACKNOWLEDGMENTS
We thank Tanya Burney for assistance with this project. This work was supported by Air Force Scientific Research Medical Free-Electron Program agreement no. AF-9550-04-1-0101, by Laser Microbeam and Medical Program, Beckman Laser Institute, University of California, Irvine (no. RR01192), and National Institutes of Health (no. 1U54NS 063718-01).
REFERENCES
- 1.Hall AH, Rumack BH. Clinical toxicology of cyanide. Ann Emerg Med. 1986;15:1067–74. doi: 10.1016/s0196-0644(86)80131-7. [DOI] [PubMed] [Google Scholar]
- 2.Baskin SI, Brewer TG. Medical aspects of chemical and biological warfare, Chapter 10, Cyanide Poisoning. In: Sidell FR, Takafuji ET, Franz DR, editors. Textbook of Military Medicine, Part I, Warfare, Weaponry, and the Casualty, pp. Borden Institute; Washington, DC: 1997. pp. 272–286. [Google Scholar]
- 3.Cummings TF. The treatment of cyanide poisoning. Occup Med (Lond) 2004;54:82–5. doi: 10.1093/occmed/kqh020. [DOI] [PubMed] [Google Scholar]
- 4.Alarie Y. Toxicity of fire smoke. Crit Rev Toxicol. 2002;32:259–89. doi: 10.1080/20024091064246. [DOI] [PubMed] [Google Scholar]
- 5.Barillo DJ, Goode R. Esch V: Cyanide poisoning in victims of fire: analysis of 364 cases and review of the literature. J Burn Care Rehabil. 1994;15:46–57. doi: 10.1097/00004630-199401000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Baud FJ, Barriot P, Toffis V, et al. Elevated blood cyanide concentrations in victims of smoke inhalation. N Engl J Med. 1991;325:1761–6. doi: 10.1056/NEJM199112193252502. [DOI] [PubMed] [Google Scholar]
- 7.Riddle K. Hydrogen cyanide: fire smoke's silent killer. JEMS. 2004;29(8)(suppl 5) [PubMed] [Google Scholar]
- 8.Strickland A, Wang RY, Hoffman RS, Goldfrank LR. Blood cyanide concentrations after smoke inhalation. N Engl J Med. 1992;326:1362. doi: 10.1056/NEJM199205143262013. [DOI] [PubMed] [Google Scholar]
- 9.Alcorta R. Smoke inhalation and acute cyanide poisoning. Hydrogen cyanide poisoning proves increasingly common in smoke-inhalation victims. JEMS. 2004;29(suppl 6-15) quiz suppl 16-7. [PubMed] [Google Scholar]
- 10.Ferrari LA, Arado MG, Giannuzzi L, Mastrantonio G. Guatelli MA: Hydrogen cyanide and carbon monoxide in blood of convicted dead in a polyurethane combustion: a proposition for the data analysis. Forensic Sci Int. 2001;121:140–3. doi: 10.1016/s0379-0738(01)00464-9. [DOI] [PubMed] [Google Scholar]
- 11.Groff WA, Sr, Stemler FW, Kaminskis A, Froehlich HL, Johnson RP. Plasma free cyanide and blood total cyanide: a rapid completely automated microdistillation assay. J Toxicol Clin Toxicol. 1985;23:133–63. doi: 10.3109/15563658508990623. [DOI] [PubMed] [Google Scholar]
- 12.Brierley JB, Prior PF, Calverley J, Brown AW. Cyanide intoxication in Macaca mulatta. Physiological and neuropathological aspects. J Neurol Sci. 1977;31:133–57. doi: 10.1016/0022-510x(77)90011-9. [DOI] [PubMed] [Google Scholar]
- 13.Brierley JB, Brown AW, Calverley J. Cyanide intoxication in the rat: physiological and neuropathological aspects. J Neurol Neurosurg Psychiatry. 1976;39:129–40. doi: 10.1136/jnnp.39.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moriya F, Hashimoto Y. Potential for error when assessing blood cyanide concentrations in fire victims. J Forensic Sci. 2001;46:1421–5. [PubMed] [Google Scholar]
- 15.Isom GE, Borowitz JL. Modification of cyanide toxicodynamics: mechanistic based antidote development. Toxicol Lett. 1995;82-83:795–9. doi: 10.1016/0378-4274(95)03521-4. [DOI] [PubMed] [Google Scholar]
- 16.Leavesley HB, Li L, Prabhakaran K, Borowitz JL, Isom GE. Interaction of cyanide and nitric oxide with cytochrome c oxidase: implications for acute cyanide toxicity. Toxicol Sci. 2008;101:101–11. doi: 10.1093/toxsci/kfm254. [DOI] [PubMed] [Google Scholar]
- 17.Alexander K, Baskin SI. The inhibition of cytochrome oxidase by diaminomaleonitrile. Biochim Biophys Acta. 1987;912:41–7. doi: 10.1016/0167-4838(87)90245-7. [DOI] [PubMed] [Google Scholar]
- 18.Egekeze JO, Oehme FW. Cyanides and their toxicity: a literature review. Tijdschr Diergeneeskd. 1980;105(suppl 2):104–14. [PubMed] [Google Scholar]
- 19.Panda M, Robinson NC. Kinetics and mechanism for the binding of HCN to cytochrome c oxidase. Biochemistry. 1995;34:10009–18. doi: 10.1021/bi00031a024. [DOI] [PubMed] [Google Scholar]
- 20.Lee PA, Sylvia AL, Piantadosi CA. Cyanide-related changes in cerebral O2 delivery and metabolism in fluorocarbon-circulaled rats. Toxicol Appl Pharmacol. 1988;94:34–44. doi: 10.1016/0041-008x(88)90334-1. [DOI] [PubMed] [Google Scholar]
- 21.Beinert H, Shaw RW, Hansen RE, Hartzell CR. Studies on the origin of the near-infrared (800-900 nm) absorption of cytochrome c oxidase. Biochim Biophys Acta. 1980;591:458–70. doi: 10.1016/0005-2728(80)90176-0. [DOI] [PubMed] [Google Scholar]
- 22.Piantadosi CA, Sylvia AL. Cerebral cytochrome a, a3 inhibition by cyanide in bloodless rats. Toxicology. 1984;33:67–79. doi: 10.1016/0300-483x(84)90017-9. [DOI] [PubMed] [Google Scholar]
- 23.Piantadosi CA, Sylvia AL, Jobsis FF. Cyanide-induced cytochrome a,a3 oxidation-reduction responses in rat brain in vivo. J Clin Invest. 1983;72:1224–33. doi: 10.1172/JCI111078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tamura M. Protective effects of a PGI2 analogue OP-2507 on hemorrhagic shock in rats–with an evaluation of the metabolic recovery using near-infrared optical monitoring. Jpn Circ J. 1992;56:366–75. doi: 10.1253/jcj.56.366. [DOI] [PubMed] [Google Scholar]
- 25.Vanneste WH. The stoichiometry and absorption spectra of components a and a-3 in cytochrome c oxidase. Biochemistry. 1966;5:838–48. doi: 10.1021/bi00867a005. [DOI] [PubMed] [Google Scholar]
- 26.Leybell I. [April 3, 2008];Toxicity, Cyanide. 2006 Available at http://www.emedicine.com/emerg/topic118.htm.
- 27.Baskin SI, Porter DW, Rockwood GA, et al. In vitro and in vivo comparison of sulfur donors as antidotes to acute cyanide intoxication. J Appl Toxicol. 1999;19:173–83. doi: 10.1002/(sici)1099-1263(199905/06)19:3<173::aid-jat556>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 28.Way JL, Leung P, Cannon E, et al. The mechanism of cyanide intoxication and its antagonism. Ciba Found Symp. 1988;140:232–43. doi: 10.1002/9780470513712.ch14. [DOI] [PubMed] [Google Scholar]
- 29.Breen PH, Isserles SA, Westley J, Roizen MF, Taitelman UZ. Effect of oxygen and sodium thiosulfate during combined carbon monoxide and cyanide poisoning. Toxicol Appl Pharmacol. 1995;134:229–34. doi: 10.1006/taap.1995.1188. [DOI] [PubMed] [Google Scholar]
- 30.Breen PH, Isserles SA, Westley J, Roizen MF. Taitelman UZ: Combined carbon monoxide and cyanide poisoning: a place for treatment. Anesth Analg. 1995;80:671–7. doi: 10.1097/00000539-199504000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Borron SW. Recognition and treatment of acute cyanide poisoning. J Emerg Nurs. 2006;32:S12–8. doi: 10.1016/j.jen.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 32.FDA [April 17, 2008];Approves Drug to Treat Cyanide Poisoning. 1996 Available at http://www.fda.gov/bbs/topics/NEWS/2006/NEW01531.html.
- 33.Branco-Ferreira M, Clode MH, Pereira-Barbosa MA, Palma-Carlos AG. Anaphylactic reaction to hydroxycobalamin. Allergy. 1997;52:118–9. doi: 10.1111/j.1398-9995.1997.tb02561.x. [DOI] [PubMed] [Google Scholar]
- 34.Hovding G. Anaphylactic reaction after injection of vitamin B12. BMJ. 1968;3:102. doi: 10.1136/bmj.3.5610.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dey LP. [July 29, 2008];Cyanokit, Napa, CA. 2006 Available at http://www.cyanokit.com/
- 36.Gulsen G, Xiong B, Birgul O, Nalcioglu O. Design and implementation of a multifrequency near-infrared diffuse optical tomography system. J Biomed Opt. 2006;11:014020. doi: 10.1117/1.2161199. [DOI] [PubMed] [Google Scholar]
- 37.Tromberg BJ, Coquoz O, Fishkin JB, et al. Non-invasive measurements of breast tissue optical properties using frequency-domain photon migration. Philos Trans R Soc Lond Ser B Biol Sci. 1997;352:661–8. doi: 10.1098/rstb.1997.0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee J, Cerussi AE, Saltzman D, Waddington T, Tromberg BJ, Brenner M. Hemoglobin measurement patterns during noninvasive diffuse optical spectroscopy monitoring of hypovolemic shock and fluid replacement. J Biomed Opt. 2007;12:024001. doi: 10.1117/1.2715189. [DOI] [PubMed] [Google Scholar]
- 39.Lee J, Saltzman DJ, Cerussi AE, et al. Broadband diffuse optical spectroscopy measurement of hemoglobin concentration during hypovolemia in rabbits. Physiol Meas. 2006;27:757–67. doi: 10.1088/0967-3334/27/8/009. [DOI] [PubMed] [Google Scholar]
- 40.Pham TH, Hornung R, Ha HP, et al. Noninvasive monitoring of hemodynamic stress using quantitative near-infrared frequency-domain photon migration spectroscopy. J Biomed Opt. 2002;7:34–44. doi: 10.1117/1.1427046. [DOI] [PubMed] [Google Scholar]
- 41.Cerussi A, Van Woerkom R, Waffarn F, Tromberg B. Noninvasive monitoring of red blood cell transfusion in very low birthweight infants using diffuse optical spectroscopy. J Biomed Opt. 2005;10:051401. doi: 10.1117/1.2080102. [DOI] [PubMed] [Google Scholar]
- 42.Lee J, El-Abaddi N, Duke A, Cerussi AE, Brenner M, Tromberg BJ. Noninvasive in vivo monitoring of methemoglobin formation and reduction with broadband diffuse optical spectroscopy. J Appl Physiol. 2006;100:615–22. doi: 10.1152/japplphysiol.00424.2004. [DOI] [PubMed] [Google Scholar]
- 43.Tromberg BJ, Coquoz O, Fishkin JB, et al. Frequency-domain photon migration (FDPM) measurements of normal and malignant cell and tissue optical properties, Biomedical Optical Spectroscopy and Diagnostics (BOSD), Orlando, FL. OSA Trends in Optics and Photonics Series. 1996;3:AP13. [Google Scholar]
- 44.Bevilacqua F, Piguet D, Marquet P, Gross JD, Tromberg BJ, Depeursinge C. In vivo local determination of tissue optical properties: applications to human brain. Appl Opt. 1999;38:4939–50. doi: 10.1364/ao.38.004939. [DOI] [PubMed] [Google Scholar]
- 45.Haskell RC, Svaasand LO, Tsay TT, Feng TC, McAdams MS, Tromberg BJ. Boundary conditions for the diffusion equation in radiative transfer. J Opt Soc Am A. 1994;11:2727–41. doi: 10.1364/josaa.11.002727. [DOI] [PubMed] [Google Scholar]
- 46.Lee J, Armstrong J, Kreuter K, Tromberg BJ, Brenner M. Non-invasive in vivo diffuse optical spectroscopy monitoring of cyanide poisoning in a rabbit model. Physiol Meas. 2007;28:1057–66. doi: 10.1088/0967-3334/28/9/007. [DOI] [PubMed] [Google Scholar]
- 47.Merritt SI, Sakurai R, Cerussi AE, Durkin AJ, Tromberg BJ. Frontiers in Optics. Optical Society of America; Rochester, NY: 2004. Monitoring deep tissue temperature with broadband diffuse optical spectroscopy. [Google Scholar]
- 48.Kwong R, Cerussi A, Tromberg B. BiOS. Society for Optical Engineering; San Jose, CA: 2007. Effects of the Range and Number of Modulation Frequencies in Diffuse Optical Spectroscopy Techniques. pp. 6434–81. [Google Scholar]
- 49.Zijlistra WG, Buursma A. Assendelft OWV: Visible and Near Infrared Absorption Spectra of Human and Animal Haemoglobin: Determination and Application. VSP; Boston, MA: 2000. [Google Scholar]
- 50.Cuccia DJ, Bevilacqua F, Durkin AJ, et al. In vivo quantification of optical contrast agent dynamics in rat tumors by use of diffuse optical spectroscopy with magnetic resonance imaging coregistration. Appl Opt. 2003;42:2940–50. doi: 10.1364/ao.42.002940. [DOI] [PubMed] [Google Scholar]
- 51.Bevilacqua F, Berger AJ, Cerussi AE, Jakubowski D, Tromberg BJ. Broadband absorption spectroscopy in turbid media by combined frequency-domain and steady-state methods. Appl Opt. 2000;39:6498–507. doi: 10.1364/ao.39.006498. [DOI] [PubMed] [Google Scholar]
- 52.Clark CJ, Campbell D, Reid WH. Blood carboxyhaemoglobin and cyanide levels in fire survivors. Lancet. 1981;1:1332–5. doi: 10.1016/s0140-6736(81)92516-2. [DOI] [PubMed] [Google Scholar]
- 53.Babcock GT, Wikstrom M. Oxygen activation and the conservation of energy in cell respiration. Nature. 1992;356:301–9. doi: 10.1038/356301a0. [DOI] [PubMed] [Google Scholar]
- 54.Jobsis-VanderVliet FF, Piantadosi CA, Sylvia AL, Lucas SK, Keizer HH. Near-infrared monitoring of cerebral oxygen sufficiency. I. Spectra of cytochrome c oxidase. Neurol Res. 1988;10:7–17. doi: 10.1080/01616412.1988.11739809. [DOI] [PubMed] [Google Scholar]
- 55.Moody AJ. ‘As prepared’ forms of fully oxidised haem/Cu terminal oxidases. Biochim Biophys Acta. 1996;1276:6–20. doi: 10.1016/0005-2728(96)00035-7. [DOI] [PubMed] [Google Scholar]