Abstract
Aim:
To assess the therapeutic effect of melatonin on heat-induced acute lung inflammation and injury in rats.
Methods:
Heatstroke was induced by exposing anesthetized rats to heat stress (36 °C, 100 min). Rats were treated with vehicle or melatonin (0.2, 1, 5 mg/kg) by intravenous administration 100 min after the initiatioin of heatstroke and were allowed to recover at room temperature (26 °C). The acute lung injury was quantified by morphological examination and by determination of the volume of pleural exudates, the number of polymorphonuclear (PMN) cells, and the myeloperoxidase (MPO) activity. The concentrations of tumor necrosis factor, interleukin (IL)-1β, IL-6, and IL-10 in bronchoalveolar fluid (BALF) were measured by ELISA. Nitric oxide (NO) level was determined by Griess method. The levels of glutamate and lactate-to-pyruvate ratio were analyzed by CMA600 microdialysis analyzer. The concentrations of hydroxyl radicals were measured by a procedure based on the hydroxylation of sodium salicylates leading to the production of 2,3-dihydroxybenzoic acid (DHBA).
Results:
Melatonin (1 and 5 mg/kg) significantly (i) prolonged the survival time of heartstroke rats (117 and 186 min vs 59 min); (ii) attenuated heatstroke-induced hyperthermia and hypotension; (iii) attenuated acute lung injury, including edema, neutrophil infiltration, and hemorrhage scores; (iv) down-regulated exudate volume, BALF PMN cell number, and MPO activity; (v) decreased the BALF levels of lung inflammation response cytokines like TNF-alpha, interleukin (IL)-1β, and IL-6 but further increased the level of an anti-inflammatory cytokine IL-10; (vi) reduced BALF levels of glutamate, lactate-to-pyruvate ratio, NO, 2,3-DHBA, and lactate dehydrogenase.
Conclusion:
Melatonin may improve the outcome of heatstroke in rats by attenuating acute lung inflammation and injury.
Keywords: free radicals, heat stroke, melatonin, acute lung injury, Wistar rats, tumor necrosis factor alpha, interleukins, nitric oxide
Introduction
Heatstroke can be defined as a systemic condition of excessive hyperthermia (body core temperature above 40 °C) associated with a systemic inflammatory response that leads to multiple organ dysfunction, predominantly in the brain (eg, delirium, convulsion, and coma)1, 2. Heatstroke is the third most common cause of fatal brain injury in the world3. More than three quarters of studied heatstroke patients develop multiple organ dysfunction, the most common of which is acute respiratory distress syndrome clinically manifested as inflammatory lung injury of very rapid onset4. Indeed, acute lung inflammation and injury can be induced in a rat heatstroke model5, 6.
Melatonin, the main product of the pineal gland, is found in high concentrations in other body fluids and tissues7, 8 and has anti-oxidant and anti-inflammatory actions9, 10, 11, 12. We have evaluated the prophylactic effect of melatonin in heatstroke rats and showed that the systemic delivery of melatonin immediately before the start of heat stress significantly prolongs the survival time of heatstroke rats13. However, it is not known whether melatonin can be used as a therapeutic agent for heatstroke resuscitation.
The present study had two purposes. First, experiments were conducted to assess the effect of melatonin administered immediately after the onset of heatstroke on the survival time of heatstroke rats. Second, we attempted to assess the temporal profile of acute lung inflammation and injury in heatstroke rats treated with melatonin or vehicle. In our previous study13, prophylactic doses of melatonin (0.2–5.0 mg/kg, intravenously) were effective in preventing the occurrence of heatstroke. The same dosage of melatonin was used in the present study to test its therapeutic action.
Materials and methods
Animals
Adult male Wistar rats (weighing 226–248 g) were obtained from the Animal Resource Center of the Republic of China National Science of Council (Taipei, Taiwan). The animals were housed four to a cage at ambient temperature (Ta) of 26±0.5 °C with a 12-h light/dark cycle. Pelleted rat chow and tap water were available ad libitum. The rats were allowed to become acclimated for at least 1 week. The experimental protocol was approved by the Animal Ethic Committee of Chi Mei Medical Center (Tainan, Taiwan) under the Guidelines of the National Science Council of the Republic of China (Taipei, Taiwan). Animal care and experiments were conducted according to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (publication No 85–23, revised 1996). In all experiments, adequate anesthesia — via a single intraperitoneal dose of urethane (1.4 g/kg of body weight) — was maintained for approximately 480 min to abolish the corneal reflex and pain reflexes induced by tail pinching. At the end of the experiments, control rats and rats that had survived heatstroke were killed with an overdose of urethane.
Surgery and monitoring of physiological parameters
The right femoral artery and vein of rats were cannulated with polyethylene tubing (PE50) under urethane anesthesia for blood pressure monitoring and drug administration, respectively. The body core temperature (Tco) was monitored continuously by a thermocouple, and mean arterial pressure (MAP) was monitored continuously with a pressure transducer.
Induction of heatstroke
The Tco of the anesthetized animals was maintained at approximately 37 °C with an infrared light lamp, except in the heat stress experiments. Heatstroke was induced by placing the animals in a folded heating pad at 36 °C controlled by circulating hot water. The time at which the MAP started to drop and the time at which MAP dropped to a value of approximately 50 mmHg were found to be 100 and 140 min, respectively, after the start of heat stress13. At 100 min, the heating pad was removed, and the animals were allowed to recover at room temperature (26 °C)13. Survival time values (the interval between the 100-min time point and death) were determined. Our previous results showed that the vehicle-treated heated rats had both hyperthermia (∼40 °C Tco) and hypotension (∼50 mmHg) at 140 min, suggesting the occurrence of heatstroke13.
Preparation of the melatonin solution
Melatonin (Sigma, St Louis, MO, USA) was dissolved in a minimum volume of ethanol (0.5 mL) and diluted to the desired concentration with normal saline. In the vehicle-treated heatstroke group, an intravenous dose of vehicle solution (1 mL of ethanol-normal saline solution per kilogram of body weight) was administered 120 min after the onset of heat stress. In the melatonin-treated heatstroke group, an intravenous dose of melatonin solution (0.2–5 mg of melatonin in 1 mL of ethanol-normal saline solution per kilogram of body weight) was administered 120 min after the start of heat stress.
Experimental groups
Animals were assigned randomly to one of three groups. The normothermic control group was treated with an intravenous dose of vehicle solution and exposed to a Ta of 26 °C. The vehicle-treated heatstroke group was treated with the same dose of vehicle solution 120 min after the initiation of heat exposure (36 °C for 100 min). The third group of rats was treated with an intravenous dose of melatonin (0.2, 1.0, or 5.0 mg/kg) solution 120 min after the onset of heat stress. The last two groups of rats were exposed to heat stress (36 °C) for exactly 100 min to induce heatstroke and were then allowed to recover at room temperature (26 °C). In all groups, the physiological parameters and survival time were observed for up to 480 min (or to the end of the experiments).
Lung morphology
At the end of the experiments, the animals were killed, and the lungs not used for lavage were excised en bloc. Lung tissues were fixed in 10% buffered formalin for 24 h and then embedded in paraffin and cut into 3-μm thick sections. Sections were stained with hematoxylin and eosin, and images were taken with an Olympus BX51 microscope with a 40×objective. The lung injury scoring method of Su et al14, 15 was modified and applied to quantify changes in lung architecture visible by light microscopy. The degree of microscopic injury was scored on the basis of the following variables: alveolar and interstitial edema, neutrophil infiltration, and hemorrhage. The severity of injury was graded for each variable: no injury=0; injury to 25% of the field=1; injury to 55% of the field=2; injury to 75% of the field=3; and diffuse injury=4. All samples were analyzed on a scaled grading system by a pathologist who was blinded to the experimental protocol and the region of sampling. A total of three slides prepared using each lung sample were randomly screened, and the mean was taken as the representative value of the sample. For presentation, we chose typical examples that were observed in all preparations of the same treatment.
Determination of the volume of pleural exudates and the number of polymorphonuclear (PMN) cells
The chest was carefully opened, and the plural cavity was washed with 2 mL of saline solution with heparin (5 U/mL) and indomethacin (10 μg/mL). The exudates and the washing solution were removed by aspiration, and the total volume was measured. The results were calculated by subtracting the volume injected (2 mL) from the total volume recovered. Cells were counted with the aid of a hemocytometer, and the PMN populations were found to contain at least 95% PMN cells as demonstrated by cytospin and differential stain analysis (vital Trypan Blue stain).
Measurements of the levels of cytokines, glutamate, lactate/pyruvate ratio, lactate dehydrogenase (LDH), nitric oxide (NO) metabolites, and 2,3-dihydroxybenzoic acid (2,3-DHBA) in bronchoalveolar fluid (BALF)
In separate experiments, the lungs were lavaged by the installation of 5 mL saline solution at room temperature through a PE tube (2.0 mm in diameter) onto the trachea. BALF was obtained 140 min after the start of heat stress or at the equivalent time for normothermic controls. The 5 mL of saline installed into the lung was withdrawn. After centrifugation at 830×g for 10 min, the BALF supernatant was collected for measurement.
The concentrations of tumor necrosis factor-alpha (TNF-α), interleukin-1β (IL-1β), IL-6, and IL-10 in the BALF were determined using the double antibody sandwich ELISA (R&D systems, Minneapolis, MN, USA) according to the manufacturer's instructions. Optical densities were read on a plate reader. The concentrations of these cytokines in the samples were calculated from the standard curve multiplied by the dilution factor and expressed as pg/mL.
To determine the glutamate levels and lactate-to-pyruvate ratio, 5-μL aliquots of the samples were injected onto a CMA600 microdialysis analyzer (Carnegie Medicine, Stockholm, Sweden) for measurement16. It should be stressed that BALF samples were not re-used for the determination of markers of oxidative/nitrosative stress.
Nitric oxide (NO) is an unstable molecule that is easily degraded into nitrite and nitrate ions17. These stable NO metabolites have been reported to reflect the levels of regional NO production/release. Nitrite and nitrate levels were measured using an HPLC-NO detector system (ENO-10; Eicom) as reported previously18. In brief, nitrite and nitrate were separated on a reverse-phase column (NO-PAK, 4.6 mm×50 mm; Eicom), and nitrate was reduced to nitrite by passage through a reduction column (NO-RED; Eicom). Nitrite was determined as the Azo Dye compound formed by the Griess reaction using a spectrophotometer. These oxidative NO products were also evaluated as NO.
The concentrations of hydroxyl radicals were measured by a modified procedure based on the hydroxylation of sodium salicylates by hydroxyl radicals leading to the production of 2,3-DHBA and 2,5-DHBA19. A Ringer's solution containing 0.5 mmol/L sodium salicylate was perfused through the microdialysis probe at a constant flow rate (1.2 μL/min). An all-time reverse phase C18 column (150 mm×1 mm internal diameter, particle size 5 μm; BAS) was used to separate the DHBA moieties, and the mobile phase consisted of a mixture of 0.1 mol/L chloroacetic acid, 26.87 nmol/L disodium EDTA, 688.16 nmol/L sodium octylsulfate and 10% acetonitrile (pH 3.0). The retention times of 2,3-DHBA and 2,5-DHBA were 8.1 and 6.0 min, respectively.
Measurements of myeloperoxidase (MPO) activity in lung tissue
MPO activity, an index of PMN cell accumulation, was determined as previously described20. Lung tissues were homogenized in a solution containing 0.5% hexa-decyl-trimethyl-ammonium bromide dissolved in 10 mmol/L potassium phosphate buffer (pH 7.0) and centrifuged for 30 min at 20 000×g at 4 °C. An aliquot of the supernatant was then allowed to react with a solution of 1.6 mmol/L tetra-methyl-benzedrine and 0.1 mmol/L H2O2. The rate of change in absorbance was measured by spectrophotometry at 650 nm. MPO activity was defined as the quantity of enzyme needed to degrade 1 μmol/mL of peroxide at 37 °C and was expressed in ng/mg protein of wet tissue.
Statistical analysis
All data are expressed as the mean±SD. A one-way analysis of variance with Tukey's multiple comparisons test was used for BALF or lung markers and physiological parameters. Significant differences were established if P<0.05. For all statistical analyses, SPSS software version 10.0 (SPSS Inc, Chicago, IL, USA) was used.
Results
Melatonin therapy attenuates hyperthermia and hypotension and improves survival during heatstroke
Table 1 summarizes the survival time for vehicle- and melatonin-treated rats during heatstroke. The melatonin-treated heatstroke rats had significantly higher survival time values compared with the vehicle-treated rats (P<0.05): the survival times were 56–62 min (n=6) for vehicle-treated rats and 178–194 min (n=6) for melatonin (5 mg/kg)-treated rats.
Table 1. Effects of heat exposure (36 °C for 100 min) on survival time in rats treated with vehicle or melatonin immediately after the initiation of heat exposure. Except for the normothermic controls, data are presented as the mean±SD. n=6. bP<0.05 compared with Group 1; eP<0.05 compared with Group 2.
| Treatment group | Survival time (min) |
|---|---|
| 1. Normothermic controls | >480 |
| 2. Vehicle-treated heatstroke rats* | 59±3b |
| 3. Melatonin (0.2 mg/kg)-treated heatstroke rats* | 65±3 |
| 4. Melatonin (1 mg/kg)-treated heatstroke rats* | 117±5e |
| 5. Melatonin (5 mg/kg)-treated heatstroke rats* | 186±8e |
Group 1 rats were sacrificed approximately 480 min after the initiation of the experiment (or at the end of the experiment) by an over-dose of anesthetic; otherwise, they should survive more than 480 min.
*For the groups exposed to 36 °C, the heat stress was withdrawn at 100 min, and the rats were allowed to recover at room temperature (26 °C).
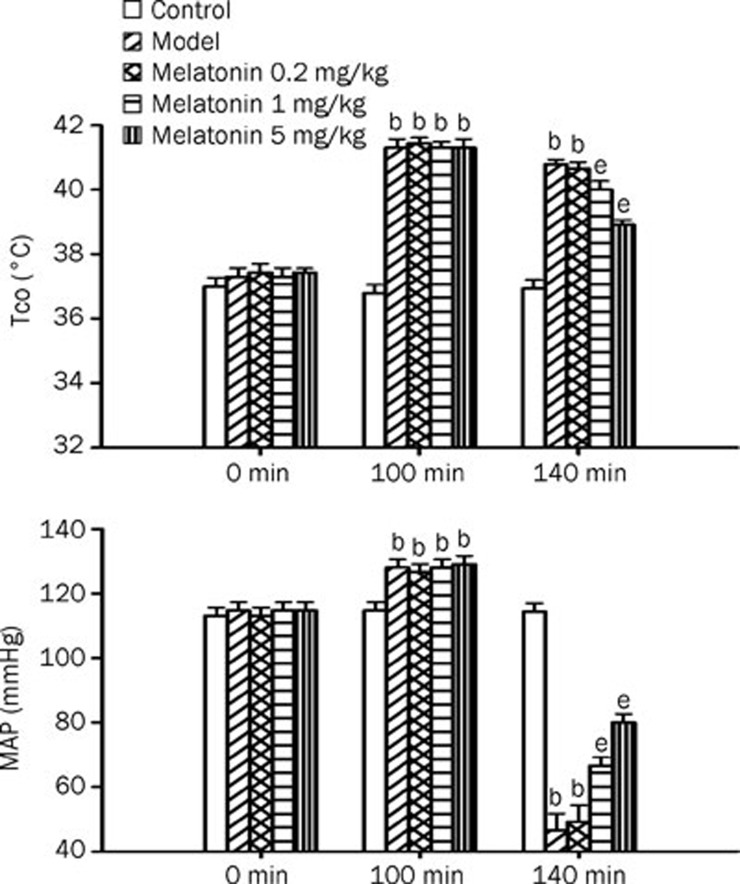
Figure 1 shows the effect of heat exposure (36 °C for 100 min) on both Tco and MAP in rats treated with vehicle or melatonin (0.2–5 mg/kg). As shown in Figure 1, 40 min after the termination of heat exposure in the vehicle-treated group, the MAP values were significantly lower than those of the normothermic controls (P<0.05). In contrast, the Tco values in the vehicle-treated heatstroke rats were significantly higher than those of the normothermic controls. Heatstroke-induced hyperthermia and hypotension were significantly and dose-dependently attenuated by melatonin therapy (1–5 mg/kg).
Figure 1.
Core temperature (Tco, A) and mean arterial pressure (MAP, B) values for normothermic controls, vehicle-treated heatstroke rats, melatonin (0.2 mg/kg)-treated heatstroke rats, melatonin (1 mg/kg)-treated heatstroke rats, and melatonin (5 mg/kg)-treated heatstroke rats. The values were obtained at 0, 100, or 140 min after the initiation of heat exposure in heatstroke rats or the equivalent times in normothermic controls. All heatstroke groups had heat exposure (36 °C) withdrawn at exactly 100 min and were then allowed to recover at room temperature (26 °C). Bars are the mean±SD of 6 rats for each group. bP<0.05 compared with normothermic controls; eP<0.05 compared with vehicle-treated heatstroke rats.
Melatonin therapy reduces acute lung injury during heatstroke
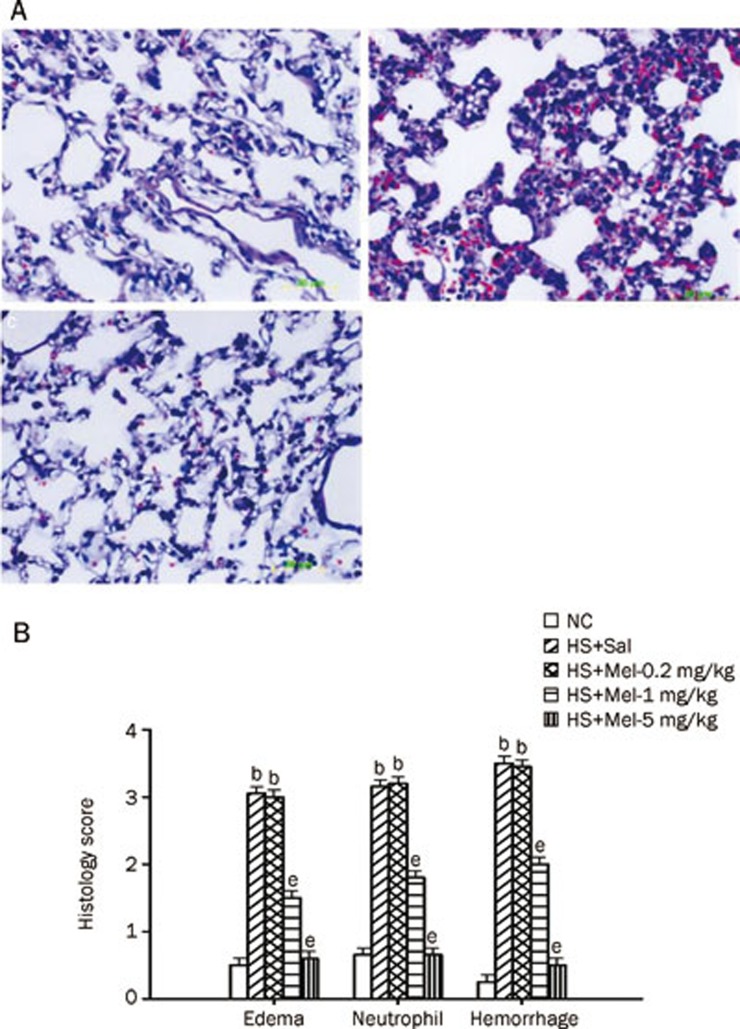
Figure 2 summarizes the effects of heat exposure on acute lung injury scores in rats treated with vehicle or with melatonin (0.2–5 mg/kg). A typical example of hematoxylin-eosin staining of the lung is depicted in Figure 2A. The images of the lungs that were not lavaged in the histology revealed that all the injury scores, including edema, neutrophil infiltration, and hemorrhage scores, were significantly increased in the vehicle-treated heatstroke group compared with the normothermic controls (Figure 2B). However, when compared with the vehicle-treated group, the animals of the melatonin (1–5 mg/kg)-treated heatstroke group had significantly lower injury scores (Figure 2B).
Figure 2.
Histological examination of lung tissue from normothermic controls (NC), vehicle-treated heatstroke rats (HS+Sal), melatonin (0.2 mg/kg)-treated heatstroke rats (HS+Mel-0.2 mg/kg), melatonin (1 mg/kg)-treated heatstroke rats (HS+Mel-1 mg/kg), and melatonin (5 mg/kg)-treated heatstroke rats (HS+Mel-5 mg/kg). (A) Representative lung microscopic image from an NC rat (a), an HS+Sal rat (b), and an HS+Mel-5 mg/kg rat (c). The HS+Sal rats had interstitial edema, neutrophil accumulation and hemorrhage. The lung pathological changes that occurred during heatstroke were significantly attenuated by melatonin (1–5 mg/kg) (P<0.05). (B) The level of edema, neutrophils infiltration, and hemorrhage score. Data are expressed as the mean±SD. n=6. bP<0.05 compared with the NC group; eP<0.05 compared with the vehicle-treated heatstroke group (HS+Sal).
Melatonin therapy down-regulates exudate volume, BALF PMN cell number, and lung MPO activity during heatstroke
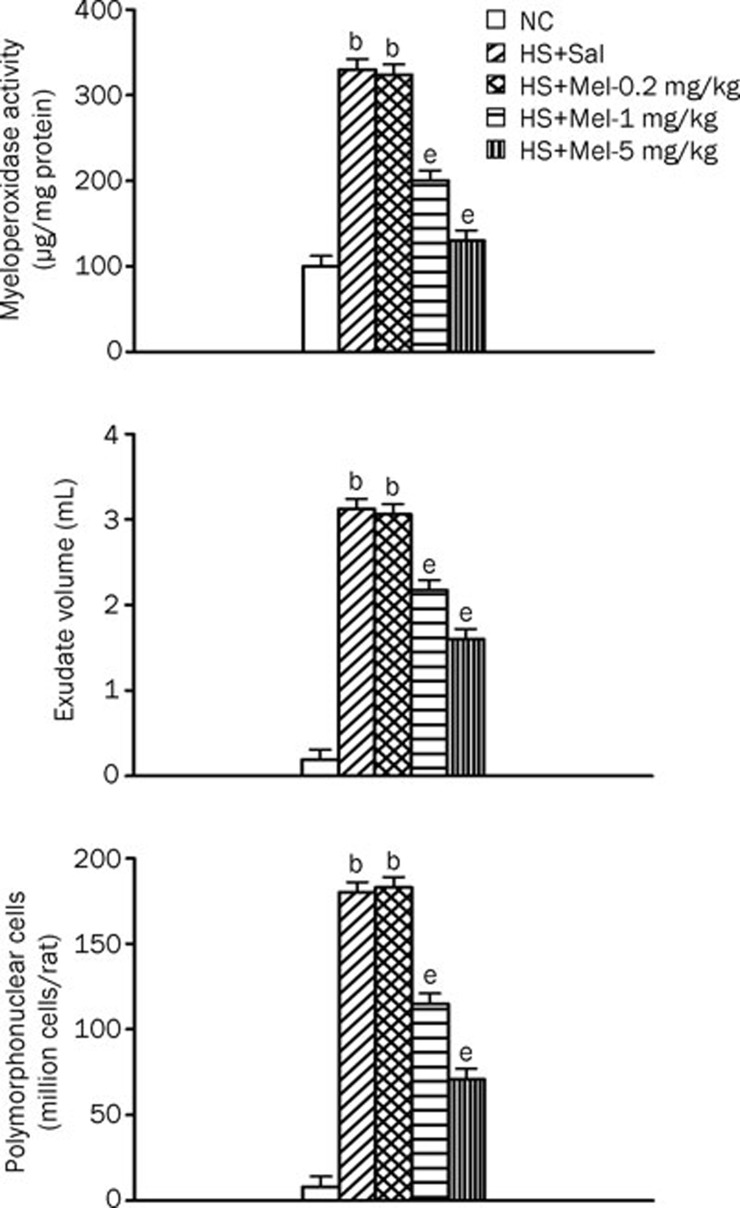
Figure 3 summarizes the effects of heat exposure on exudate volume, the number of PMN cells in the BALF, and lung MPO activity of normothermic controls, vehicle-treated heatstroke rats, and melatonin (0.2–5 mg/kg)-treated heatstroke rats. At 140 min after the start of heat exposure, the exudate volume, number of PMN cells in the BALF, and the lung MPO activity were greater in vehicle-treated heatstroke rats than in the normothermic controls. However, all these indicators of acute pleurisy were greatly attenuated by melatonin therapy.
Figure 3.
Exudate volume, number of PMN cells and MPO activity in lung tissues from NC, HS+Sal, HS+Mel (0.2 mg/kg), HS+Mel (1 mg/kg), and HS+Mel (5 mg/kg) rats. Data are expressed as mean±SD. n=6. bP<0.05 compared with the NC group; eP<0.05 compared with the HS+Sal group.
Melatonin up-regulates BALF IL-10 levels but down-regulates BALF TNF-α, IL-1β, and IL-6 levels during heatstroke
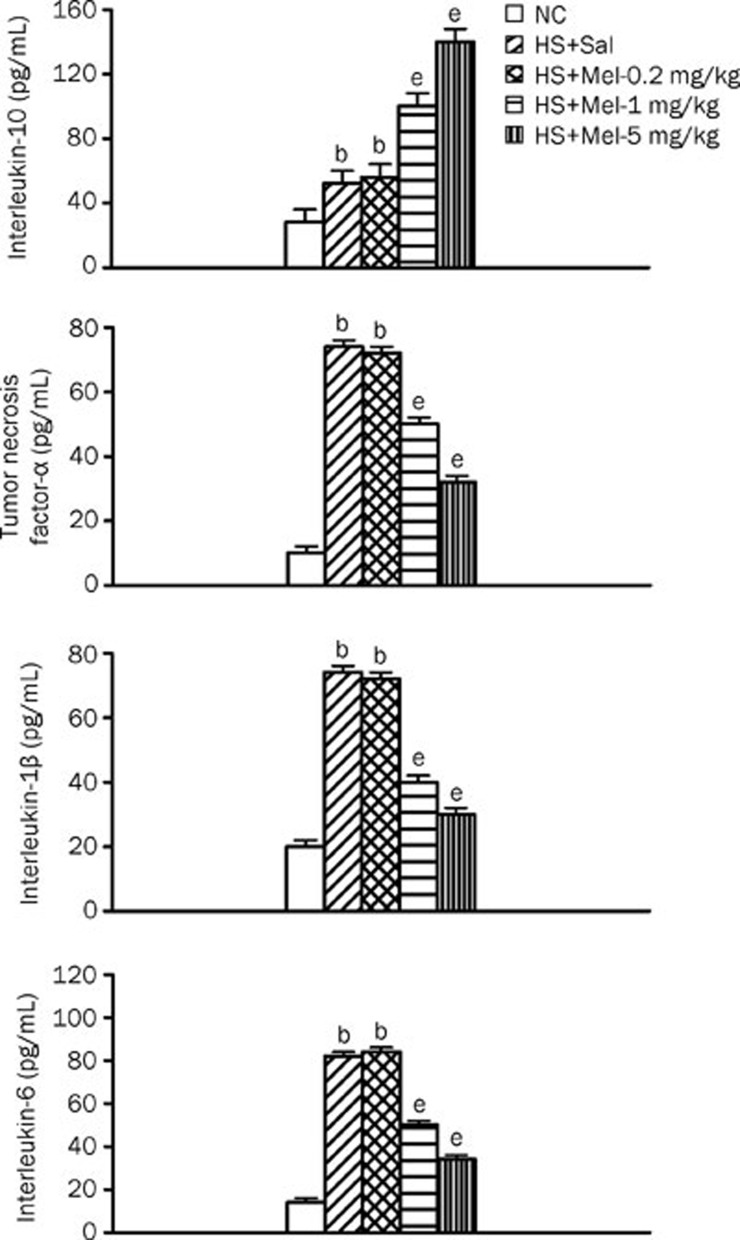
Figure 4 summarizes the BALF IL-10, TNF-α, IL-1β, and IL-6 levels among the three experimental groups. Compared with the normothermic controls, vehicle-treated heatstroke rats had significantly higher levels of IL-10, TNF-α, IL-1β, and IL-6 at 140 min after the onset of heat stress. The increase in the BALF levels of TNF-α, IL-1β, and IL-6 during heatstroke was significantly reduced by melatonin therapy (1–5 mg/kg). However, compared with vehicle-treated rats, melatonin therapy (1–5 mg/kg) further enhanced the BALF levels of heat-induced IL-10.
Figure 4.
Interleukin-10, tumor necrosis factor-alpha, interleukin-1β, and interleukin-6 levels in BALF from the NC, HS+Sal, HS+Mel (0.2 mg/kg), HS+Mel (1 mg/kg), and HS+Mel (5 mg/kg) rat. Data are expressed as the mean±SD. n=6. bP<0.05 compared with the NC group; eP<0.05 compared with the HS+Sal group.
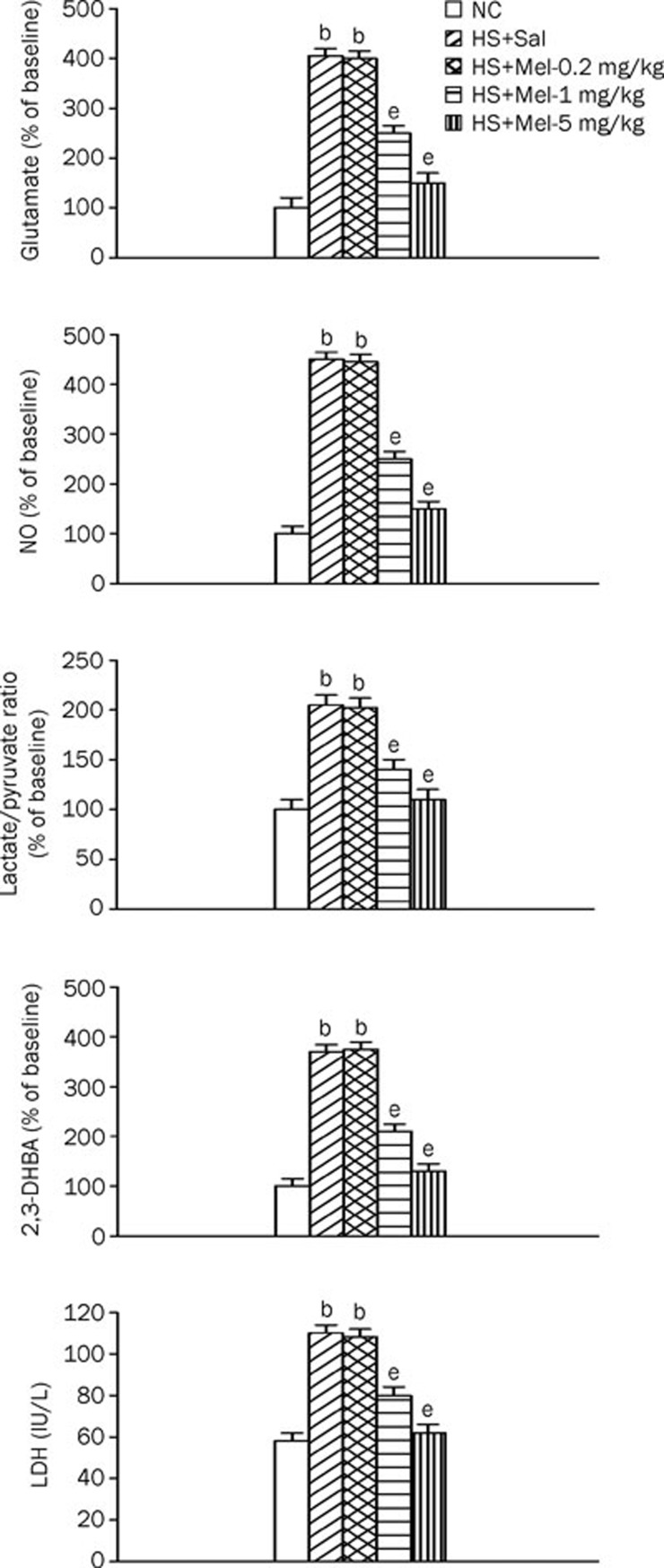
Melatonin therapy down-regulates the BAL levels of glutamate, NO, lactate-to-pyruvate ratio, 2,3-DHBA, and LDH during heatstroke
Figure 5 shows the levels of glutamate, NO, lactate-to-pyruvate ratio, 2,3-DHBA, and LDH in the BALF among the three experimental groups. Compared with normothermic controls, vehicle-treated heatstroke rats had significantly higher levels of all these markers 140 min after the start of heat stress. These increases were significantly reduced by melatonin therapy (1–5 mg/kg).
Figure 5.
Glutamate, lactate-to-pyruvate ratio, lactate dehydrogenase (LDH), nitric oxide metabolites (NO), and 2,3-dihydroxybenzoic acid (2,3-DHBA) levels in BALF from the NC, HS+Sal, HS+Mel (0.2 mg/kg), HS+Mel (1 mg/kg), and HS+Mel (5 mg/kg) rats. Data are expressed the meanSD. n=6. bP<0.05 compared with the NC group; eP<0.05 compared with the HS+Sal group.
Discussion
On the level of the whole organism, severe heat stress (42–43 °C for ∼70 min) caused hyperthermia (i.e., body core temperature >40 °C), hypotension (mean arterial pressure <50 mmHg), intracranial hypertension, splanchnic vasoconstriction, and hypoxia, which might facilitate the leakage of endotoxin from the intestine to the systemic circulation and result in excessive activation of PMN cells and endothelial cells1, 2, 21. In this study, mild heat stress (36 °C for 100 min) also caused hyperthermia, hypotension, and many aspects of heatstroke reactions, described below. This indicates that the MAP value of ∼50 mmHg and hyperthermia (>40 °C body core temperature) used in the present study are sufficient to represent heat shock.
Endotoxin causes multiple organ dysfunction, including acute lung injury22. During acute lung injury, acute lung inflammation is characterized by the local recruitment and activation of PMNs23 and the release of proinflammatory mediators24, 25, proteases, and reactive oxygen and nitrogen species26, 27. These reactions lead to alveolar-capillary damage, with high permeability lung edema and alteration of lung mechanics, and result in severe gas exchange abnormalities28. Endotoxin can stimulate the overproduction of a variety of proinflammatory mediators, such as TNF-α, IL-1β, IL-6, and NO29. Concurrently, IL-10, an anti-inflammatory cytokine, is induced to limit the net inflammatory response in the lungs30. The pleiotropic transcription factor nuclear-kappa B (NF-κB) also plays a crucial role in regulating the expression of cytokines, chemokines, adhesion molecules, and other mediators22, 31.
Like endotoxin-induced septic shock, heatstroke rats showed (i) increased lung activity of MPO (an indicator for PMN cell infiltration); (ii) increased exudate volume and PMN cell numbers (indicators of acute pleurisy); (iii) increased BALF levels of proinflammatory cytokines, such as TNF-α and IL-1β, and the anti-inflammatory cytokine IL-10; (iv) increased BALF levels of glutamate and the lactate-to-pyruvate ratio (cellular ischemia markers), the toxic oxidizing radicals NO and 2,3-DHBA, and LDH (an indicator of toxic organ injury); and (v) increased acute lung edema, neutrophil infiltration, and hemorrhage scores.
This study provides the first data showing the beneficial effects of melatonin on acute lung inflammation and injury in heatstroke rats. Our results clearly indicate that melatonin treatment significantly (i) reduced acute lung injury, including edema, neutrophil infiltration, and hemorrhage scores; (ii) decreased acute pleurisy; (iii) decreased BALF levels of proinflammatory cytokines, a cellular ischemia marker, and ischemic and oxidative damage markers; and (iv) reduced lung MPO activity in rats during heatstroke. In conclusion, heatstroke causes acute lung inflammation and injury, and melatonin has a beneficial effect against acute lung inflammation and injury in heatstroke. Therefore, melatonin has a therapeutic potential in the clinical treatment of lung inflammation and injury.
Our results are partly consistent with previous findings obtained from different disease models. For example, melatonin reduces acute lung injury in endotoxemic rats22 or in sleep-deprived mice32by attenuating pulmonary inflammation and inhibiting NF-κB activation22. Melatonin protects against acute lung injury caused by radiation therapy in a rat model26. Additionally, oral melatonin attenuates acute lung injury caused by the inhalation of aerosolized pancreatic fluids in rats by reducing lung inflammation and airway hyperactivity10. Leukocyte rolling and adhesion to the microcirculation in rats is inhibited by melatonin27. Melatonin attenuates paraquet-induced acute lung toxicity in rats by inhibiting oxidative damage28. Melatonin is efficient in ameliorating ischemia/reperfusion injury of the intestine and lung in rats by inhibiting both oxidative and nitrosative stress in these organs33. Together, these observations led us to conclude that melatonin might attenuate heatstroke-induced acute lung injury in rats by inhibiting pulmonary inflammation, NF-κB activation, and oxidative and nitrosative stress in the lung. Of course, the hypothesis warrants further verification in future studies.
It has been shown that the BALF levels of pro-inflammatory cytokines, such as IL-1β, are elevated in patients with adult respiratory distress syndrome34. An overproduction of TNF-α is also noted in endotoxin-induced lung inflammation35. Both our previous6 and present results confirm that the inflammatory process (eg, heat-induced acute pleurisy) leads to the overproduction of pro-inflammatory cytokines, such as TNF-alpha, IL-1β, and IL-6, in the BALF. Our results further show that the increased BALF levels of both IL-1β and TNF-α during experimental heatstroke can be reduced by melatonin. In addition, BALF IL-10 levels in heatstroke are elevated by melatonin. IL-10 can attenuate both IL-1β and TNF-α production36. Therefore, melatonin may attenuate acute lung inflammation during heatstroke by stimulating IL-10 production and inhibiting the production of proinflammatory cytokines, including IL-1β and TNF-α, in the BALF.
The above-mentioned pro-inflammatory cytokines lead to the release of large amounts of the toxic oxidizing radicals, such as NO, superoxide anion (O2−), and hydroxyl radicals, from neutrophils, macrophages, and monocytes, which causes multiple organ injury via the peroxidation of membrane lipids, the oxidative damage of proteins and DNA37, or the augmentation of local tissue injury38. This systemic inflammatory response would result in the sequestration of polymorphonuclear cells in the lung and liver, which would lead to vascular dysfunction and multiple organ dysfunction39. In the present study, melatonin therapy may have caused the attenuation of acute lung injury in heatstroke by reducing the overproduction of pro-inflammatory cytokines, toxic oxidizing radicals, the sequestration of polymorphonuclear cells, and organ damage markers in lung tissues.
Both glutamate and the lactate-to-pyruvate ratio are markers of cellular ischemia40, 41. Our data show that the excessive elevation of both glutamate levels and the lactate-to-pyruvate ratio in the BALF occurred during heatstroke, suggesting that heat stress causes lung ischemia. We further demonstrate that heat-induced lung ischemia can be attenuated by melatonin.
It should be stressed that our previous results demonstrated that systemic administration of melatonin immediately before the onset of heatstroke significantly prolongs the survival time of rats13. In the present study, the delivery of melatonin immediately after the onset of heatstroke also significantly prolonged survival time. The results indicate that melatonin can be used as both a prophylactic and a therapeutic agent for experimental heatstroke. As shown by our previous and present results, the time to death of heatstroke rats treated with melatonin was prolonged, but all the animals eventually died. In fact, these animals were under general urethane anesthesia. To verify that there was durable improvement in survival, unanesthetized, unrestrained animals with or without melatonin treatment should be exposed to heat stress.
In summary, the present study provides the data showing that the occurrence of pulmonary edema, inflammation, and ischemic and oxidative damage caused by heatstroke in rats can be attenuated by melatonin therapy.
Key messages
Heatstroke rats display acute lung edema, inflammation, and injury. Melatonin therapy significantly attenuated heat-induced acute lung edema, inflammation, and injury.
Abbreviations
Tco, core body temperature; Ta, ambient temperature; MAP, mean arterial pressure; PMN, polymorphonuclear; LDH, lactate dehydrogenase; NO, nitric oxide; DHBA, dihydroxybenzoic acid; BALF, bronchoalveolar fluid; TNF-α, tumor necrosis factor-alpha; IL-1β, interleukin-1β IL-6, interleukin-6; IL-10, interleukin-10; MPO, myeloperoxidase.
Author contribution
Mao-tsun LIN and Ching-ping CHANG helped to draft the manuscript or to critically revise it. Furthermore, Chen-kuei CHANG, Mao-tsun LIN, Wen-shiann WU, Ming-ting CHOU,and Chien-ming CHAO participated in the design, coordination and data acquisition of the study. Wen-shiann WU and Ching-ping CHANG participated in the statistical analysis.
References
- Bouchama A, Knochel JP. Heat stroke. N Engl J Med. 2002;346:1978–88. doi: 10.1056/NEJMra011089. [DOI] [PubMed] [Google Scholar]
- Chang CK, Chang CP, Chiu WT, Lin MT. Prevention and repair of circulatory shock and cerebral ischemia/injury by various agents in experimental heatstroke. Curr Med Chem. 2006;13:3145–54. doi: 10.2174/092986706778742945. [DOI] [PubMed] [Google Scholar]
- Sharma HS, Westman J.Brain function in hot environment Progress in brain research 115Elsevier, Amsterdam, 1998p 1–516. [Google Scholar]
- Varghese GM, John G, Thomas K, Abraham OC, Mathai D. Predictors of multi-organ dysfunction in heatstroke. Emerg Med J. 2005;22:185–7. doi: 10.1136/emj.2003.009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HH, Chang CP, Cheng JT, Lin MT. Inhibition of acute lung inflammation and injury is a target of brain cooling after heatstroke injury. J Trauma. 2010;69:805–12. doi: 10.1097/TA.0b013e3181cb43fd. [DOI] [PubMed] [Google Scholar]
- Yang HH, Chang CP, Cheng JT, Lin MT. Attenuation of acute lung inflammation and injury by whole body cooling in a rat heatstroke model. J Biomed Biotechnol. 2009;2009:768086. doi: 10.1155/2009/768086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menendez-Pelaez A, Reiter RJ. Distribution of melatonin in mammalian tissues: the relative importance of nuclear versus cytosolic localization. J Pineal Res. 1993;15:59–69. doi: 10.1111/j.1600-079x.1993.tb00511.x. [DOI] [PubMed] [Google Scholar]
- Reiter RJ, Tan DX. What constitutes a physiological concentration of melatonin. J Pincal Res. 2000;34:79–80. doi: 10.1034/j.1600-079x.2003.2e114.x. [DOI] [PubMed] [Google Scholar]
- Galano A, Tan DX, Reiter RJ. Melatonin as a natural ally against stress: a physicochemical examination. J Pineal Res. 2011;51:1–16. doi: 10.1111/j.1600-079X.2011.00916.x. [DOI] [PubMed] [Google Scholar]
- Chen CF, Wang D, Reiter RJ, Yeh DY. Oral melatonin attenuates lung inflammation and airway hyperreactivity induced by inhalation of aerosolized pancreatic fluid in rats. J Pineal Res. 2011;50:46–53. doi: 10.1111/j.1600-079X.2010.00808.x. [DOI] [PubMed] [Google Scholar]
- Manda K, Ueno M, Anzai K. AFMK, a melatonin metabolite, attenuates X-ray-induced oxidative damage to DNA, proteins and lipids in mice. J Pineal Res. 2007;42:386–93. doi: 10.1111/j.1600-079X.2007.00432.x. [DOI] [PubMed] [Google Scholar]
- Hardeland R, Backhaus C, Fadavi A. Reactions of the NO redox forms NO+, *NO and HNO (protonated NO-) with the melatonin metabolite N1-acetyl-5-methoxykynuramine. J Pineal Res. 2007;43:382–8. doi: 10.1111/j.1600-079X.2007.00489.x. [DOI] [PubMed] [Google Scholar]
- Lin XJ, Mei GP, Liu J, Li YL, Zuo D, Liu SJ, et al. Therapeutic effects of melatonin on heatstroke-induced multiple organ dysfunction syndrome in rats. J Pineal Res. 2011;50:436–44. doi: 10.1111/j.1600-079X.2011.00863.x. [DOI] [PubMed] [Google Scholar]
- Li Z, Gao C, Wang Y, Liu F, Ma L, Deng C, et al. Reducing pulmonary injury by hyperbaric oxygen preconditioning during simulated high altitude exposure in rats. J Trauma. 2011;71:673–9. doi: 10.1097/TA.0b013e3181f5b073. [DOI] [PubMed] [Google Scholar]
- Su X, Bai C, Hong Q, Zhu D, He L, Wu J, et al. Effect of continuous hemofiltration on hemodynamics, lung inflammation and pulmonary edema in a canine model of acute lung injury. Intensive Care Med. 2003;29:2034–42. doi: 10.1007/s00134-003-2017-3. [DOI] [PubMed] [Google Scholar]
- Chou YT, Lai ST, Lee CC, Lin MT. Hypothermia attenuates circulatory shock and cerebral ischemia in experimental heatstroke. Shock. 2003;19:388–93. doi: 10.1097/00024382-200304000-00016. [DOI] [PubMed] [Google Scholar]
- Yamada K, Nabeshima T. Simultaneous measurement of nitrite and nitrate levels as indices of nitric oxide release in the cerebellum of conscious rats. J Neurochem. 1997;68:1234–43. doi: 10.1046/j.1471-4159.1997.68031234.x. [DOI] [PubMed] [Google Scholar]
- Togashi H, Mori K, Ueno K, Matsumoto M, Suda N, Saito H, et al. Consecutive evaluation of nitric oxide production after transient cerebral ischemia in the rats hippocampus using in vivo brain microdialysis. Neurosci Lett. 1998;240:53–7. doi: 10.1016/s0304-3940(97)00918-x. [DOI] [PubMed] [Google Scholar]
- Yang CY, Lin MT. Oxidative stress in rats with heatstroke-induced cerebral ischemia. Stroke. 2002;33:790–4. doi: 10.1161/hs0102.100208. [DOI] [PubMed] [Google Scholar]
- Mullane KM, Kraemer R, Smith B. Myeloperoxidase activity as a quantitative assessment of neutrophil infiltration into ischemic myocardium. J Pharmacol Methods. 1985;14:157–67. doi: 10.1016/0160-5402(85)90029-4. [DOI] [PubMed] [Google Scholar]
- Hall DM, Buettner GR, Oberley LW, Xu L, Matthes RD, Gisolfi CV. Mechanisms of circulatory and intestinal barrier dysfunction during whole body hyperthermia. Am J Physiol Heart Circ Physiol. 2001;280:H509–21. doi: 10.1152/ajpheart.2001.280.2.H509. [DOI] [PubMed] [Google Scholar]
- Shang Y, Xu SP, Wu Y, Jiang YX, Wu ZY, Yuan SY, et al. Melatonin reduces acute lung injury in endotoxemic rats. Chin Med J. 2009;122:1388–93. [PubMed] [Google Scholar]
- Chignard M, Balloy V. Neutrophil recruitment and increased permeability during acute lung injury induced by lipopolysaccharide. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1083–90. doi: 10.1152/ajplung.2000.279.6.L1083. [DOI] [PubMed] [Google Scholar]
- Wright RM, Ginger LA, Kosila N, Elkins ND, Essary B, McManaman JL, et al. Mononuclear phagocyte xanthine oxidoreductase contributes to cytokine-induced acute lung injury. Am J Respir Cell Mol Biol. 2004;30:479–90. doi: 10.1165/rcmb.2003-0309OC. [DOI] [PubMed] [Google Scholar]
- Shinbori T, Walczak H, Krammer PH. Activated T killer cells induce apoptosis in lung epithelial cells and the release of pro-inflammatory cytokine TNF-alpha. Eur J Immunol. 2004;34:1762–70. doi: 10.1002/eji.200425097. [DOI] [PubMed] [Google Scholar]
- Haddad IY, Pataki G, Hu P, Galliani C, Beckman JS, Matalon S. Quantitation of nitrotyrosine levels in lung sections of patients and animals with acute lung injury. J Clin Invest. 1994;94:2407–13. doi: 10.1172/JCI117607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthay MA, Geiser T, Matalon S, Ischiropoulos H. Oxidant-mediated lung injury in the acute respiratory distress syndrome. Crit Care Med. 1999;27:2028–30. doi: 10.1097/00003246-199909000-00055. [DOI] [PubMed] [Google Scholar]
- Liu D, Zeng BX, Shang Y. Decreased expression of peroxisome proliferator-activated receptor gamma in endotoxin-induced acute lung injury. Physiol Res. 2006;55:291–9. doi: 10.33549/physiolres.930822. [DOI] [PubMed] [Google Scholar]
- Liu D, Zeng BX, Zhang SH, Wang YL, Zeng L, Geng ZL, et al. Rosiglitazone, a peroxisome proliferator-activated receptor-gamma agonist, reduces acute lung injury in endotoxemic rats. Crit Care Med. 2005;33:2309–16. doi: 10.1097/01.ccm.0000183161.81503.7d. [DOI] [PubMed] [Google Scholar]
- Pedreira PR, García-Prieto E, Parra D, Astudillo A, Diaz E, Taboada F, et al. Effects of melatonin in an experimental model of ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2008;295:L820–7. doi: 10.1152/ajplung.90211.2008. [DOI] [PubMed] [Google Scholar]
- Ali S, Mann DA. Signal transduction via the NF-kappaB pathway: a targeted treatment modality for infection, inflammation and repair. Cell Biochem Funct. 2004;22:67–79. doi: 10.1002/cbf.1082. [DOI] [PubMed] [Google Scholar]
- Lee YD, Kim JY, Lee KH, Kwak YJ, Lee SK, Kim OS, et al. Melatonin attenuates lipopolysaccharide-induced acute lung inflammation in sleep-deprived mice. J Pineal Res. 2009;46:53–7. doi: 10.1111/j.1600-079X.2008.00621.x. [DOI] [PubMed] [Google Scholar]
- Kesik V, Guven A, Vurucu S, Tunc T, Uysal B, Gundogdu G, et al. Melatonin and 1400 W ameliorate both intestinal and remote organ injury following mesenteric ischemia/reperfusion. J Surg Res. 2009;157:e97–105. doi: 10.1016/j.jss.2008.12.024. [DOI] [PubMed] [Google Scholar]
- Pugin J, Ricou B, Steinberg KP, Suter PM, Martin TR. Proinflammatory activity in bronchoalveolar lavage fluids from patients with ARDS, a prominent role for interleukin-1. Am J Resp Crit Care Med. 1996;153:1850–6. doi: 10.1164/ajrccm.153.6.8665045. [DOI] [PubMed] [Google Scholar]
- Xing Z, Jordana M, Kirpalani H, Driscoll KE, Schall TJ, Gauldie J. Cytokine expression by neutrophils and macrophages in vivo: endotoxin induces tumor necrosis factor-alpha, macrophage inflammatory protein-2, interleukin-1 beta, and interleukin-6 but not RANTES or transforming growth factor-beta 1 mRNA expression in acute lung inflammation. Am J Resp Cell Molec Biol. 1994;10:148–53. doi: 10.1165/ajrcmb.10.2.8110470. [DOI] [PubMed] [Google Scholar]
- Oberholzer A, Oberholtz C, Moldawer LL. Cytokine signaling-regulation of the immune response in normal and critically ill states. Crit Care Med. 2000;28:N3–12. doi: 10.1097/00003246-200004001-00002. [DOI] [PubMed] [Google Scholar]
- Feihl F, Wasber B, Liaudet L. Is nitric oxide overproduction the target of choice for the management of septic shock. Pharmacol Ther. 2001;91:179–213. doi: 10.1016/s0163-7258(01)00155-3. [DOI] [PubMed] [Google Scholar]
- Kim PK, Deutscheman CS. Inflammatory responses and mediators. Surg Clin North Am. 2000;80:885–94. doi: 10.1016/s0039-6109(05)70102-x. [DOI] [PubMed] [Google Scholar]
- Neviere RR, Cepinskas G, Madorin WS, Hoque N, Karmazyn M, Sibbald WJ, et al. LPS pretreatment ameliorates peritonitis-induced myocardial inflammation and dysfunction: role of myocytes. Am J Physiol. 1999;277:H885–92. doi: 10.1152/ajpheart.1999.277.3.H885. [DOI] [PubMed] [Google Scholar]
- Persson L, Hillered L. Chemical monitoring of neurosurgical intensive care patients using intracerebral microdialysis. J Neurosurg. 1992;76:72–80. doi: 10.3171/jns.1992.76.1.0072. [DOI] [PubMed] [Google Scholar]
- Hillered L, Persson L. Neurochemical monitoring of the acutely injured human brain. Scand J Clin Lab Invest Suppl. 1999;229:9–18. doi: 10.1080/00365519950185904. [DOI] [PubMed] [Google Scholar]