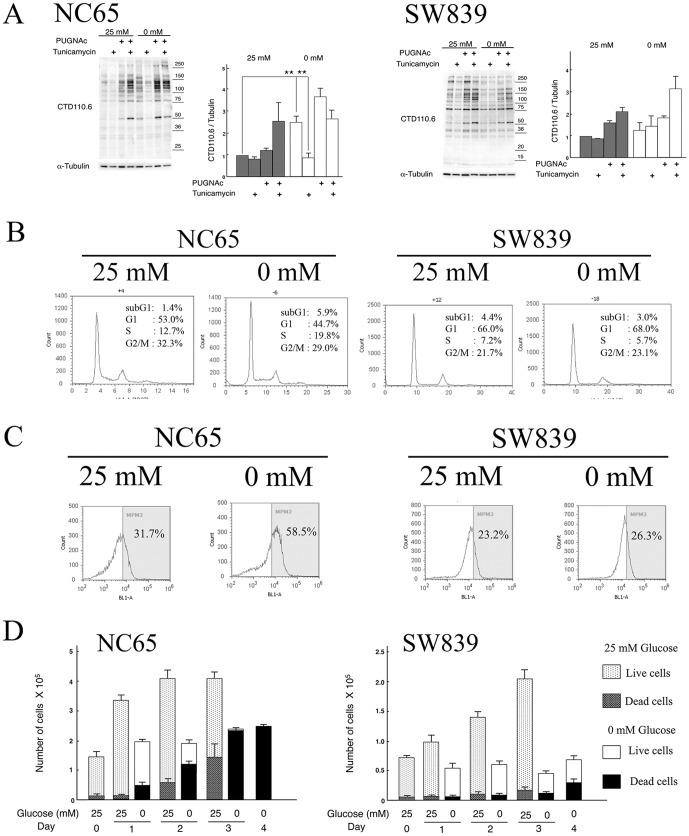
Figure 1. Characterization of NC65 and SW839 cells.
A, Immunoblot analysis. NC65 and SW839 cells were seeded in high-glucose medium and then the culture medium was replaced on day 2 with fresh high-glucose medium (25 mM glucose) or with glucose-deprived medium (0 mM glucose) for 24 h. Treatment with PUGNAc (100 µM), an inhibitor of β-D-N-acetylglucosaminase (O-GlcNAcase), and tunicamycin (2 µg/ml), an inhibitor of N-glycosylation, were carried out on when the medium was replaced. For each cell type: left panel, immunoblot using CTD110.6 and anti-α-tubulin antibodies; right panel, quantitative analysis of reactivity with the CTD110.6 antibody, normalized against the anti-α-tubulin signal for untreated cells grown in high-glucose medium (25 mM glucose). An anti-α-tubulin antibody was used as an internal control. Error bars represent standard error from three independent experiments. ** represents p<0.01. B-C, Flow cytometric analysis. NC65 and SW839 cells were cultured in fresh 25 mM or 0 mM glucose medium for 24 h after 2 days of culture in 25 mM glucose medium and then fixed and stained using FxCycle Violet nuclear staining reagent (B) and anti-MPM-2 antibody (C). D, Cell growth. The numbers of living and dead cells were counted using the trypan-blue exclusion assay on 0–4 days after the fresh medium replacement on day 2. Note that glucose deprivation of NC65 cells induced the production of N-GlcNAc2-modified proteins and G2/M transition arrest, leading to cell death. However, glucose deprivation of SW839 cells did not induce the production of N-GlcNAc2-modified proteins, and induced G1/S transition arrest, thereby allowing cell survival. Glucose deprivation also enhanced the level of MPM-2 in NC65 cells, but did not in SW839 cells.