Figure 3. Repressive chromatin modifiers involved in disorders of brain function.
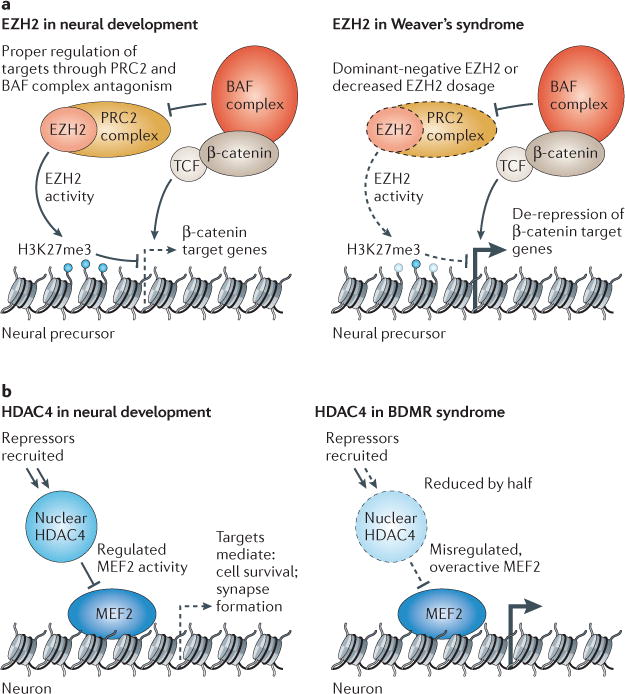
a | During later stages of neurogenesis, enhancer of zeste 2 (EZH2) has been shown to repress particular β-catenin target genes in neural progenitors in order to mediate proper cell fate transitions. It is also likely that EZH2 and BAF complexes have antagonistic roles in these cells as they do in embryonic stem cells and Drosophila melanogaster. Decreased functional EZH2 dosage (owing to haploinsufficiency or altered function of mutant proteins) will lead to de-repression or over-expression of its targets, leading to altered developmental pathways. Patients with Weaver’s syndrome have macrocephaly and learning disabilities of varying severity. b | In normal development, histone deacetylase 4 (HDAC4) is dynamically regulated in the cell, moving into and out of the nucleus in response to physiological signals. When localized in the nucleus, HDAC4 binds myocyte-specific enhancer factor 2 (MEF2) transcription factors and recruits repressors such as class I HDACs and heterochromatin protein 1 (HP1) to MEF2 targets. HDAC4 dosage or nuclear residence is critically affected in patients with brachydactyly mental retardation (BDMR) syndrome, probably leading to misregulated MEF2 target gene expression in particular temporal and cellular contexts. H3K27me3, histone H3 trimethylated at lysine 27; PRC2, Polycomb repressive complex 2.
