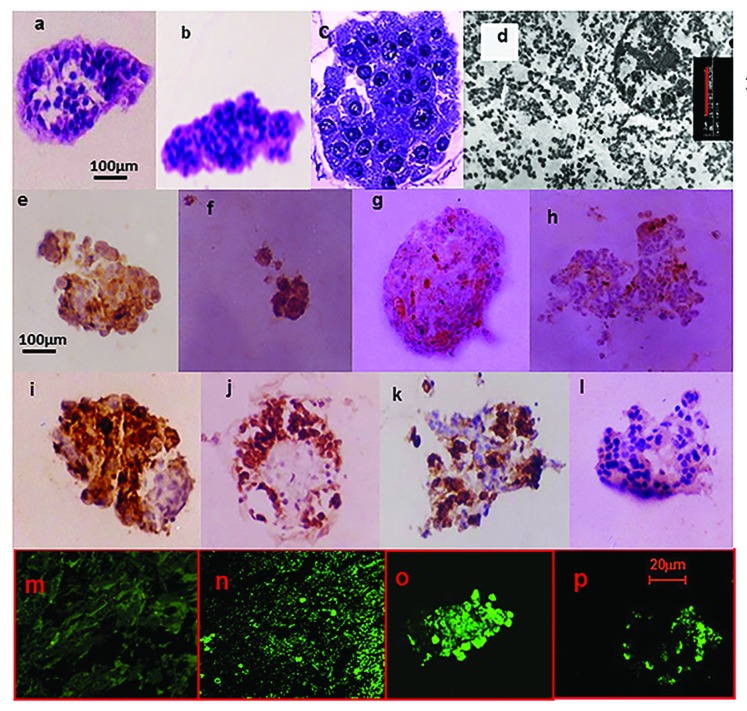
Figure 1. Histomorphology studies on post-thaw cultured isolated human islets. Physical integrity: 320× Light Microscopy 5 μm sections H&E stain of islets processed using (A) the Pre-Edmonton protocol and (B) Edmonton protocol. The islets were not contaminated by the presence of acinar tissue in both cases, illustrating the efficiency of the isolation procedure. With increasing culture duration, there was an increasing loss of cohesivity. Islets that retained their physical integrity (mantle or core was intact) exhibited cytoplasmic and nuclear appearance synonymous with normal cellular structure. While such islets were visible on all days of culture, their frequency steadily declined with culture duration. (C) 1000×—Zoom 2 semi-thin sections toluidine blue stain of islets processed using the Edmonton protocol. There was regional damage to the intra-islet extra-cellular matrix (ECM) (as illustrated). Nevertheless, cytoplasmic and nuclear integrity was retained. (D) β-cell ultra-structure uranylacetate and lead citrate stain of islets processed using the Edmonton protocol. β-cell granules were visible, though there was loss of the halo; ribosomes were present though detached; lysosomes had not yet disintegrated; nuclear, mitochondria, and plasma membrane integrity were also retained. Functionality: Immunohistochemistry (IHC) for the islet hormones (x320). By days 5 and 7, there were increasing number of ghost cells that answered positive for more than one of the islet hormones. IHC for Insulin (E); Glucagon (F); Somatostatin (G); and PP (H) islets processed using the Pre-Edmonton protocol. IHC for Insulin (I); Glucagon (J); Somatostatin (K); and PP (L) islets processed using the Edmonton protocol. Apoptosis: Confocal Microscopy In Situ Cell Death (Apoptosis) Detection Kit, Terminal Uridine Nucleotide End Labeling (TUNEL)-Roche. Fluorescein labeling: (M) negative control, (N) positive control, (O) islets processed using the Pre-Edmonton protocol, (P) islets processed using the Edmonton protocol.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.