Abstract
Islet transplantation is a promising treatment for Type 1 diabetes; however limitations of the intra-portal site and poor revascularization of islets must be overcome. We hypothesize that engineering a highly vascularized collagen-based construct will allow islet graft survival and function in alternative sites. In this study, we developed such a collagen-based biomaterial. Neonatal porcine islets (NPIs) were embedded in collagen matrices crosslinked with 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide and N-hydroxysuccinimide containing combinations of chondroitin-6-sulfate, chitosan, and laminin, and compared with controls cultured in standard media. Islets were examined for insulin secretory activity after 24 h and 4 d and for apoptotic cell death and matrix integrity after 7 d in vitro. These same NPI/collagen constructs were transplanted subcutaneously in immunoincompetent B6.Rag−/− mice and then assessed for islet survival and vascularization. At all time points assessed during in vitro culture there were no significant differences in insulin secretory activity between control islets and those embedded in the collagen constructs, indicating that the collagen matrix had no adverse effect on islet function. Less cell death was observed in the matrix with all co-polymers compared with the other matrices tested. Immunohistochemical analysis of the grafts post-transplant confirmed the presence of intact insulin-positive islets; grafts were also shown to be vascularized by von Willebrand factor staining. This study demonstrates that a collagen, chondroitin-6-sulfate, chitosan, and laminin matrix supports islet function in vitro and moreover allows islet survival and vascularization post-transplantation; therefore, this bio-engineered vascularized construct is capable of supporting islet survival.
Keywords: islet transplantation, cell delivery, diabetes, vascularization, collagen, chitosan, chondroitin
Introduction
Since 2000, when the “Edmonton Protocol” achieved insulin independence in 7 patients by using islets from multiple donors and steroid-free anti-rejection therapy, islet transplantation has been seen as an increasingly promising treatment for patients with type 1 diabetes.1-4 Despite remarkable progress in clinical islet transplantation, the liver implantation site remains far from ideal.5-9 Although alternative sites have been suggested, including subcutaneous and intramuscular spaces, poor blood supply in these sites results in delayed graft revascularization and thereby loss of islet cell mass and function.6 Our group has previously used collagen-based biomaterial matrices to facilitate cell delivery resulting in revascularization of ischemic tissue.10,11 Such a collagen-based matrix could potentially be used to deliver islets to an alternate site, then support islet survival and function through rapid neovascularization.
Collagen is a highly biocompatible polymer and natural extracellular matrix that has been widely examined as a potential biomaterial for vascularization and islet delivery. Collagen and other polymers are commonly considered because they can be engineered to have similar properties to the extracellular matrix, allowing vascularization and cell support. Various approaches have been explored, including using a polyethylene terephthalate mesh bag around a collagen sponge to deliver Sprague-Dawley rat islets intramuscularly in Lewis rats;12 a polytetrafluoroethylene “solid support” in collagen delivering isogenic rat islets in a peritoneal site;13 and polyurethane foam filled with heparin and collagen delivering syngeneic rat islets in the subcutaneous space.14 These devices utilize a combination of natural and synthetic polymers, which is advantageous because of the ease of manufacturing and the control of mechanical properties of the synthetic polymers. Polymers are widely used as biomaterials for cell delivery and inducing vascularization because of their potential to mimic the natural extracellular matrix. However, natural polymers, compared with synthetic polymers, have improved biocompatibility of both the biomaterial and the degradation byproducts, thereby being less immunogenic. The ideal device for islet delivery should then be based on natural polymers such as collagen but also have the mechanical properties of synthetic polymers.
The poor mechanical properties resulting from the purification process of collagen necessitate modifications in order to restore mechanical strength. Two common methods to achieve this goal are chemical crosslinking and the addition of copolymers. The crosslinker 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) combined with N-hydroxysuccinimide (NHS) has been used to achieve this with minimal negative impact on cell viability.11,15,17 The addition of copolymers such as chitosan, chondroitin and laminin to the matrix can also lead to improved mechanical strength and local angiogenesis.16,17 Chitosan, prepared from the deacetylation of chitin, is a biocompatible polymer that has been shown to accelerate wound healing and vascularization.16 Crosslinking chitosan to collagen may improve the resistance of the collagen-based matrix to enzymatic degradation. Chitosan also possesses unique tissue-adhesive and antimicrobial properties that could improve the properties of the matrix.16 Collagen-chitosan constructs are successful cell delivery vehicles that promote neovascularization.16 Chondroitin-6-sulfate is a sulfated glycosaminoglycan that interacts with functional proteins to bind growth factors into the biomaterial.17 Chondroitin additionally ameliorates the matrix structure by regulating matrix elasticity, allowing collagen-chondroitin-6-sulfate gels to be utilized as potential islet delivery devices.16,18,19 Laminin, an important basement membrane protein, may aid in the retention of transplanted islets in vivo in addition to acting as a guidance molecule for vascularization and islet survival.20,21
The objective of this study was to examine the effect of various collagen-based matrices on the in vitro function of NPIs and their survival following transplantation in the subcutaneous space of mice. Neonatal porcine islets (NPIs) were used because they are a potential clinical source of islets for treating patients with type 1 diabetes.22,23
Results
In vitro assessment of islet: Collagen matrices
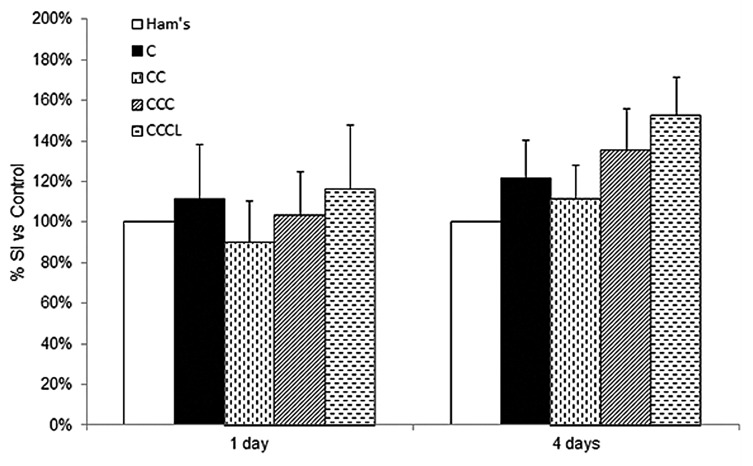
Prior to conducting the transplant studies we assessed the glucose stimulated insulin secretion of collagen embedded NPIs in order to examine if the matrices had an adverse effect. The insulin secretory capacities of NPIs embedded in the different collagen-based matrices and cultured for 1 and 4 d were tested by comparing the amount of cellular insulin that was released at low glucose (2.8 mM) and high glucose (20.0 mM). No statistically significant differences were noted in the amounts of insulin secreted at low or high glucose when comparing all collagen conditions with control NPIs. Similarly, when the stimulation indices were calculated no significant differences were observed in any conditions at either 1 d or 4 d post culture (Fig. 1). The stimulation indices shown in Figure 1 are typical of immature NPIs.
Figure 1. Glucose stimulated insulin secretions of control and collagen embedded NPIs were measured following 1 and 4 d in culture. The stimulation indices (SI) were determined by dividing the amount of insulin released at high glucose (20.0 mM) by that at low glucose (2.8 mM). The stimulation indices were subsequently normalized to the non-embedded control values. The stimulation indices for the control Ham's F10 condition after 1 and 4 d in culture were 1.42 ± 0.48 and 1.05 ± 0.18, respectively. Data are expressed as mean ± SEM (n = 6 per time point).
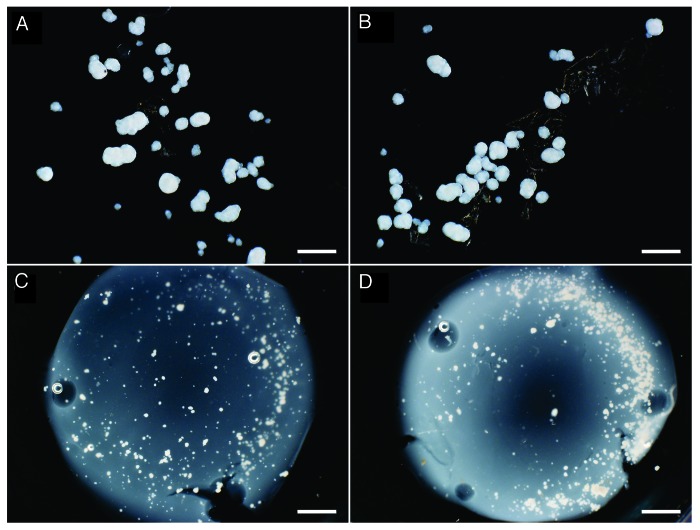
Differences in the mechanical properties were noted between the various matrices. When NPIs were added to matrices containing collagen alone (C) or collagen and chitosan (CC), the matrices did not crosslink completely. Culture media added on top of these C and CC matrices accelerated the degradation of the matrices after 7 d, leaving small pieces of matrices and loose NPIs (Fig. 2A and B). The CCC matrices did crosslink with the addition of NPIs and appeared similar to the CCCL matrices after 7 d in culture (Fig. 2C and D).
Figure 2. NPIs embedded in C (A), CC (B), CCC (C) and CCCL (D) matrices were cultured for 7 d then dark field images were taken of the matrices in a 6 well plate. Scale bars are 400 µm for A and B and 1.6 mm for C and D.
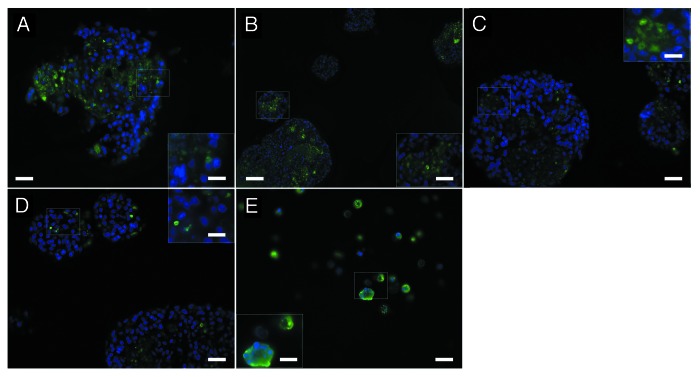
To determine the effect of the matrices on islet survival, matrices containing NPIs were also cultured for 7 d then stained for insulin and using a TUNEL assay for apoptosis (Fig. 3). Since the C and CC matrices did not adequately crosslink, we embedded the NPIs with the remaining separated pieces of the matrix in agarose. While all matrices showed intact islets, the CCCL matrix (Fig. 3D) had significantly fewer apoptotic cells with 4.3 ± 0.2% of the DAPI positive cells staining for TUNEL compared with the C matrix at 9.9 ± 0.4% (P < 0.05), the CC matrix at 7.3 ± 0.2% (P < 0.05), and the CCC matrix at 7.2 ± 0.2% (P < 0.05) (Fig. 3A, B, and C, respectively). Some of the cells stained positively for TUNEL but not for DAPI, likely indicating apoptotic cells with extremely fragmented DNA.24
Figure 3. NPIs embedded in C (A), CC (B), CCC (C) and CCCL (D) matrices following 7 d in culture were fixed and paraffin-embedded, then sections were stained for TUNEL and compared with TUNEL positive controls (E). Scale bars of A-E are 20 µm and scale bars of insets are 10 µm.
In vivo assessment of islet: Collagen matrices
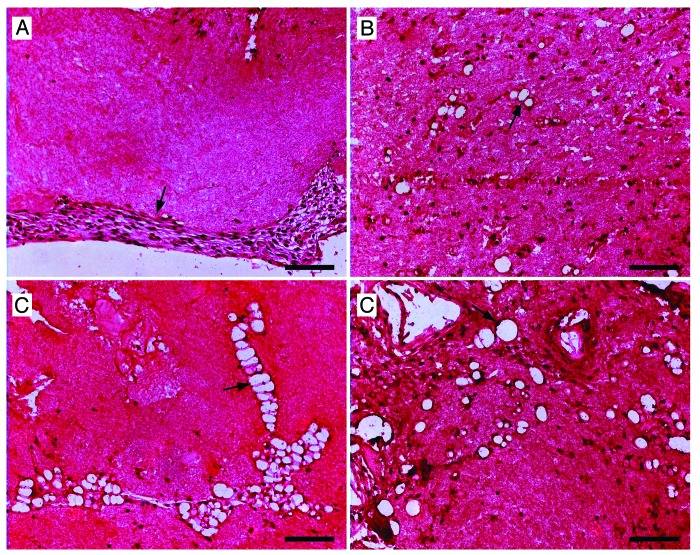
Based on the above data, the C and CC matrices were determined to be inferior and were no longer considered as potential biomaterials for islet transplantation. The CCCL matrix exhibited no adverse effects on mechanical stability, islet survival, or islet function. Chondroitin-6-sulfate binds functional proteins,16 chitosan improves vascularization, and laminin enhances vascularization20 and islet survival;21 therefore, the following in vivo transplant studies were conducted using the CCCL matrix. In a first series of experiments we implanted the CCCL matrices without NPIs subcutaneously into BALB/c mice in order to examine their potential to become vascularized. Upon retrieval, the matrices were adhered to the hypodermis and maintained their size and shape over the 28 d period. A thin, transparent membrane formed around the transplanted matrix by 4 d after transplantation. Retrieved matrices were fixed and paraffin embedded, and sections were stained for von Willebrand factor (vWF) to evaluate the potential for vascularization of the matrix. At 7 d, vWF positive endothelial cells began to penetrate the periphery of the matrix (Fig. 4A). Two weeks post-transplantation, small capillaries with a vWF positive lining were visible, migrating toward the interior of the matrix (Fig. 4B). After quantification of capillary size, these early capillaries were found to have a mean size of 53.54 ± 0.75 µm2. At 3 weeks, the capillaries increased in size to a mean area of 75.24 ± 2.32 µm2 as well as in number, and some appeared to be combining into larger structures (Fig. 4C). After 4 weeks, the capillaries were widespread throughout the matrix (Fig. 4D) and had grown to a significantly larger mean size of 221.68 ± 5.63 µm2 (P < 0.05). These data clearly demonstrate that the CCCL matrix can become vascularized following subcutaneous implantation.
Figure 4. In order to examine whether CCCL matrices without islets can be vascularized in vivo the CCCL matrixes were implanted subcutaneously in immunoincompetent BALB/c mice and retrieved after 7 (A), 14 (B), 21 (C) and 28 d (D). The grafts were fixed and paraffin-embedded; sections were stained for von Willebrand Factor (vWF), hemotoxylin, and eosin. All scale bars are 100 µm. Arrows indicate vWF positive cells (A) and capillaries (B–D).
After confirming the ability of the CCCL matrix to become vascularized, C, CC, and CCCL matrices containing NPIs were transplanted subcutaneously in naïve immunoincompetent B6.Rag−/− mice and retrieved after 4 (CCCL only), 7, 14, 21, and 28 d and examined for gross morphology (Fig. 5). The CCCL matrices were transplanted using a microspatula, but because the C and CC matrices did not crosslink sufficiently to be manipulated, they were injected using a large orifice pipette. Prior to implantation, NPIs were distributed throughout the CCCL matrix (Fig. 5A). Following transplantation, blood vessels were observed in the periphery of the matrices as early as four days (Fig. 5B) as well as at 7 and 28 d (Figs. 5C and D). However, following implantation NPIs were only observed at 4 d since the matrixes become overgrown with connective tissue making it difficult to detect the NPIs within the matrix. At 7 d and later, no C or CC matrices were found; it is likely that these matrices degraded quickly due to their lack of mechanical integrity at the time of transplantation.
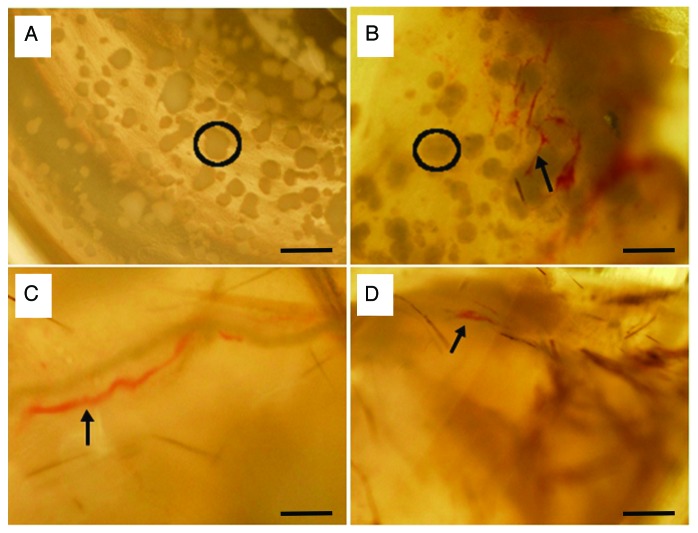
Figure 5. Gross morphology of NPIs in the CCCL gels and transplanted subcutaneously in immunocomprimised B6.Rag−/− mice. Grafts were analyzed pre-transplant (A) and after 4 (B), 21 (C) and 28 (D) days. Circles indicate visible islets; arrows indication visible vasculature. Scale bars are 400 µm.
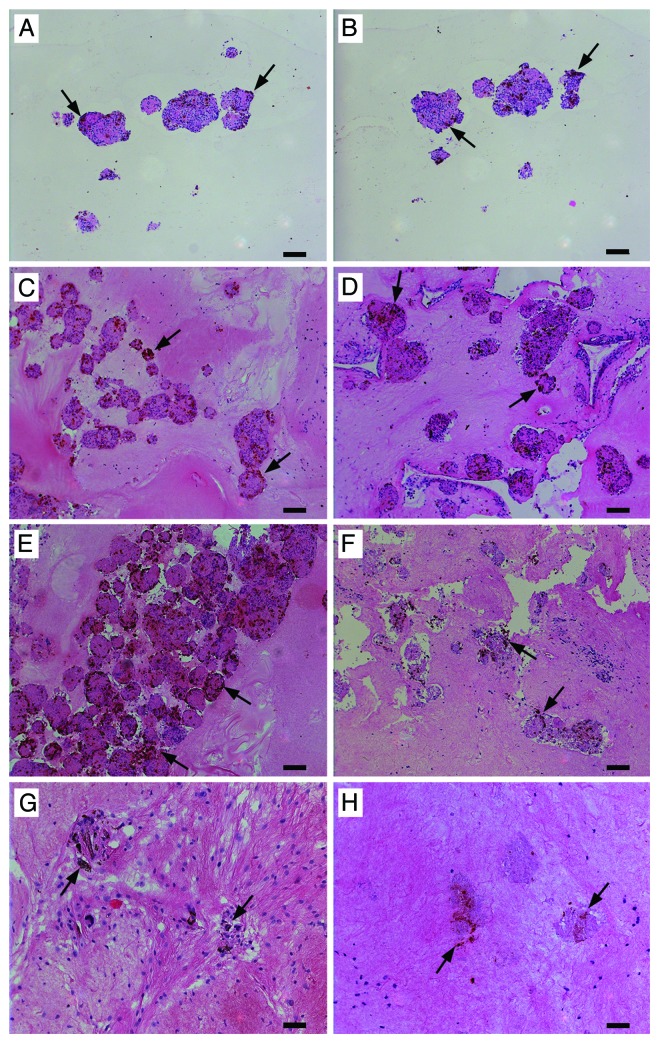
We therefore conducted another series of CCCL:NPI transplants in non-diabetic B6.Rag−/− mice and collected the grafts for histological assessment. It should be noted that since NPIs are not fully developed islets the number of insulin and glucagon positive cells are lower than that observed in adult islets. When examined for the presence of insulin and glucagon positive cells, the proportion of insulin and glucagon positive cells tended to increase at 4 d (Fig. 6C and D) and 7 d (Fig. 6E and F) post-transplantation, as compared with those prior to transplantation.
Figure 6. Neonatal porcine islets in the CCCL gels were transplanted subcutaneously and retrieved after 4 (C and D), 7 (E and F), and 28 (G and H) days. Pre-transplantation grafts were also collected (A and B). The grafts were fixed and paraffin-embedded; sections were stained for insulin, hemotoxylin and eosin (A, C, E, and G) and glucagon, hemotoxylin and eosin (B, D, F, and H). Arrows indicated insulin (A, C, E, and G) or glucagon (B, D, F, and H) positive cells. Scale bars for A-F are 100 µm and for G and H are 50 µm.
Discussion
This study demonstrates that our collagen-based matrix has the potential to create a site for islet transplantation. The insulin secretory capacity of NPIs seeded into the CCCL matrices was not adversely affected and when transplanted subcutaneously the NPIs were shown to survive as the matrix became more vascularized. When compared with controls cultured in HAM’S F10, insulin secretion of NPIs in all tested collagen matrices was comparable; the stimulation indices of the collagen embedded NPIs tended to be higher than controls most notably in CCCL, however this was not significantly different. Nonetheless these data demonstrate that the matrices did not have an adverse effect on NPI viability as indicated by comparable stimulation indices. The transplanted CCCL matrices were also shown to support NPI survival in vivo for up to 28 d as evidenced by the positive insulin and glucagon staining at each time point. There were no signs of inflammation or adverse reaction to the matrices, and the matrix maintained its size and shape over the 28 d period indicating a slow biodegradation rate. Islet survival over a longer term must still be investigated further.
A critical feature of using a collagen-based matrix for islet transplantation is being able to maintain mechanical stability. Mechanical stability is essential for physical manipulation of the matrix in order to successfully place it into the transplant site. Although this collagen based matrix is similar to that described previously by Suuronen et al.20,26 to recruit and deliver endothelial progenitor cells, our matrix required alteration to support NPIs. In this study we observed that collagen alone and collagen with chitosan matrices degraded quickly during culture upon the addition of NPIs before crosslinking and thereby lost their mechanical stability. Therefore, we had to improve the integrity of the CCCL matrix and its ability to crosslink upon the addition of NPIs. Differences from the previously described matrix20,26 include using pure rat tail collagen instead of porcine collagen; using a higher concentration of chondroitin-6-sulfate; using 20 µg/mL of laminin instead of 40 µg/mL; and using 10x DMEM and 10x HEPES as a “collagen buffer” instead of 2-(N-morpholino) ethanesulfonic acid buffer to dissolve the crosslinking chemicals.
When the co-polymer chondroitin-6-sulfate was added these matrices had improved mechanical stability, thus allowing for ease of implantation into the subcutaneous space. Additionally, the CCCL matrix better supported islet viability, as shown by fewer apoptotic cells compared with the C, CC, and CCC matrices. Because chitosan, chondroitin-6-sulfate, and laminin have beneficial properties such as binding functional proteins (chondroitin-6-sulfate) and enhancement of vascularization and islet survival (laminin), we decided to use the CCCL matrix for the subsequent in vivo transplant studies. To confirm this result, C and CC matrices were transplanted subcutaneously; as early as seven days, no C matrices were found in any of the mice and only one very small CC matrix was found with no visible islets. This indicates that the C and CC matrices, in addition to being much more difficult to transplant, biodegrade more quickly than the CCCL matrix and are therefore not suitable as biomaterials for NPI transplantation.
Our data demonstrate that at 4 d post-transplant vWF positive endothelial cells begin to penetrate the periphery of the CCCL matrix and as time progresses capillaries begin to form then subsequently increase in size and number. These data clearly demonstrate that the CCCL matrices can become highly vascularized, allowing for NPI survival. Although it is unknown if these capillaries are sufficient to support longer-term islet survival, other components may be added to the matrix such as vascular endothelial growth factor (VEGF) to further promote the growth of larger blood vessels. VEGF has previously been shown to increase vascularization in collagen-based matrices, and it is known to bind to chondroitin-6-sulfate.14,24 Stromal cell-derived factor-1 (SDF-1) is an alternative growth factor that has also been shown to improve recruitment of angiogenic cells in a collagen-based matrix in vivo.25
Another potential benefit of a CCCL matrix is that it could theoretically allow for the co-localization of ancillary cells such as mesenchymal stem cells (MSCs) with the islets. MSCs have been shown to promote vascularization in various models, including in a collagen-glycosaminoglycan scaffold wherein the mesenchymal stem cells were observed to adopt an endothelial phenotype, thereby enhancing vascularization.27 Moreover, MSCs have been also shown to have beneficial effects when co-transplanted with islets, such as promoting graft revascularization, prevention of islet allograft rejection and protection of human islets from pro-inflammatory cytokines.28-31 Because the kidney is not an applicable site for clinical islet transplantation and the liver will not ensure close association of the co-grafted cells, an alternative site must be developed. CCCL matrixes may be the solution because they would greatly improve the likelihood that the cells are co-localized.
Although islets can be supported in a collagen-based matrix as it becomes vascularized over time, it would be ideal to transplant the islets into an environment with an already established vascular system. Two ways to create this environment would be to transplant islets into a prevascularized collagen-based matrix, or to increase vascularization in the surrounding site so that there are more vessels available to grow into the matrix. Both these approaches have successfully been used in other bioengineered devices, with both natural and synthetic polymers, with prevascularization periods ranging from 14 to 60 d.12,13,24,32-34 Both approaches have also been combined with growth factors such as fibroblast growth factor (FGF)12,13,33 and vascular endothelial growth factor (VEGF).24 Prevascularizing the matrix could also be achieved by transplanting a stem cell population to promote angiogenesis. One or a combination of these approaches will be investigated further in order to optimize the conditions for islet survival and function.
Although the subcutaneous space may not be a practical site for clinical islet transplantation, it was used in this study to examine the feasibility of using the collagen matrix. A more practical site would be the omental pouch; however, the size of the collagen matrices did not permit us to use this site in mice. The ideal site for clinical islet transplantation should be optimal for islet survival and function. It should allow for portal drainage of secreted hormones without direct exposure to high concentrations of immunosuppressive drugs, host platelets and complement; accommodate a large tissue volume; be easily accessible; and allow for retrieval of the grafted tissue. The omental pouch satisfies all of these criteria and has been used to successfully cure diabetes in both rats and dogs.35-38 This site will be investigated in the future in a larger rodent model, such as the rat.
We have shown that embedding NPIs in a collagen-based matrix containing the co-polymers chitosan, chondroitin-6-sulfate and laminin does not adversely affect NPI function in vitro; when these matrices are implanted subcutaneously in mice they induced significant vascularization and the supported the survival of the NPIs. This suggests that the CCCL matrix maybe used to create a site for islet transplantation, which could include several benefits including prevention of tissue loss and cellular co-transplantation. Further study in diabetic recipients is warranted.
Materials and Methods
Preparation of neonatal porcine islets
Porcine pancreases were obtained from 1 to 3 d old Duroc neonatal piglets from the University of Alberta Swine Research Centre (1.5–2.0 kg body weight), and the islets were isolated and cultured for 5–7 d as described previously.22 Briefly, the retrieved pancreases were cut into 1 to 3 mm tissue fragments, then exposed to 2.5 mg/mL collagenase (type XI; Sigma, C7657), filtered through a 500 µm nylon screen, washed in Hank’s Basic Salt Solution (HBSS) (Gibco, H6136) supplemented with 0.25% BSA (fraction V; Sigma-Aldrich, A9543), 10 mM HEPES (Sigma-Aldrich, H4034), 100 U/mL penicillin, and 0.1 mg/mL streptomycin (Lonza Walkersville, Inc., 09-757F). NPI were then cultured in non-tissue culture treated petri dishes containing HAM’S F10 tissue culture media purchased from Sigma-Adrich (N6635), and supplemented with 14.3 mM sodium bicarbonate (Fisher, S233), 10 mM D-glucose (EM Science, DX0145-3), 2 mM L-glutamine (Sigma-Aldrich, G8540), 0.25% BSA (fraction V; Sigma-Aldrich, A9543), 50 µM isobutylmethylxanthine (IBMX) (Sigma-Aldrich, I5879), 10 mM nicotinamide (Sigma-Aldrich, N0636), 1.6 mM calcium chloride dihydrate (Sigma-Aldrich, C7902), 100 U/mL penicillin, and 0.1 mg/mL streptomycin (Lonza Walkersville, Inc., 09-757F). The islets were cultured at 37 °C for 5–7 d, with the medium changed the first, third and fifth days after isolation.
Preparation of collagen matrices
Neonatal porcine islets (NPIs) were embedded in collagen-based matrices utilizing rat tail type one collagen (BD Biosciences, Inc., 354249) crosslinked with 30 mM 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC, Sigma-Aldrich, E6383) and 30 mM N-hydroxysuccinimide (NHS, Sigma-Aldrich, 56480) containing combinations of chondroitin-6-sulfate (Wako Pure Chemical Industries, 034-14612), chitosan (Sigma-Aldrich, C3646), and mouse laminin (BD Biosciences, Inc., 354232), as outlined in Table 1. In Table 1, the acronym C refers to gels with collagen, CC collagen with 5% v/v chitosan, CCC collagen with 5% v/v chitosan and 40% w/v chondroitin, and CCCL collagen, chitosan, chondroitin and 20 µg/mL laminin. Following embedding in the various collagen matrices, islets were cultured overnight in supplemented HAM’S F10. Controls included non-embedded islets cultured in supplemented HAM’S F10 (NPIs).
Table 1. Composition of matrices tested on neonatal porcine islets.
| Matrix Composition | ||||
| Material | C | CC | CCC | CCCL |
| Collagen (mg/mL) | 6.1 | 6.0 | 5.3 | 5.2 |
| Chitosan (mg/mL) | - | 0.2 | 0.2 | 0.2 |
| Chondroitin(mg/mL) | - | - | 1.1 | 1.0 |
| Laminin (mg/mL) | - | - | - | 0.1 |
| EDC (mM) | 31 | 30 | 26 | 26 |
| NHS (mM) | 35 | 34 | 30 | 30 |
A summary of the composition of matrix types tested and their respective polymer concentration. C, collagen; CC, collagen and chitosan; CCC, collagen, chitosan, and chondroitin, and CCCL, collagen, chitosan, chondroitin, and laminin.
In vitro assessment of islets and matrices
A static incubation assay22 was used to assess glucose stimulated insulin secretion of collagen embedded and control NPIs following 1 and 4 d culture. Each condition was incubated in duplicate at 37 °C for 2 h in 1.5 mL RPMI (Roswell Park Memorial Institute medium) supplemented with 2.0 mM L-glutamine, 0.5% w/v BSA and either 2.8 mM (low) or 20.0 mM (high) glucose. The media was then separately collected and assayed for respective insulin contents by a rat insulin immunoassay that quantitatively detects porcine insulin (Meso Scale Discovery, K152BZC). Stimulation indices (SI) were calculated by dividing the amount of insulin released at 20.0 mM glucose by that released at 2.8 mM glucose. The stimulation indices were subsequently normalized to the non-embedded control values and expressed as a percentage of the control SI.
Matrices containing NPIs were cultured for 7 d in HAM’S F10 culture media supplemented as above, then dark field images were taken to identify changes in mechanical integrity. The matrices were then collected and fixed in Shandon Zinc Formal-Fixx (Thermo Fisher Scientific, 6764255) if possible; if the matrices did not have sufficient integrity to be collected on their own, the liquid matrix and NPIs were collected, fixed for 24 h in 1% formalin in PBS, and then embedded in agarose plugs. Apoptosis of the NPIs in the matrices was assessed using a commercial TUNEL assay kit (Invitrogen Molecular Probes A23210). After rehydration, antigen retrieval was performed in sodium citrate buffer (pH 6.0), then slides were incubated according to the kit instructions. The slides were mounted with ProLong Gold Antifade Reagent with DAPI (Invitrogen Molecular Probes P36935). To quantify the percentage of TUNEL positive cells, five images of TUNEL and DAPI staining were taken then the images were combined in ImageJ (National Institutes of Health). Separate images of the TUNEL and DAPI positive channels were altered to black and white, then the number of particles counted. Each particle was confirmed to be TUNEL or DAPI positive visually and the brightness of the color threshold adjusted to only include positive cells. This process was repeated by 2 independent viewers.
In vivo assessment of matrices and islets with matrices
Based on the in vitro data only the CCCL matrices were assessed in subsequent in vivo experiments. First, in order to examine whether CCCL matrices alone can be vascularized in vivo CCCL matrices were prepared without islets and transplanted subcutaneously in naïve BALB/c mice. Weekly up to 28 d these grafts were retrieved and examined for evidence of vascularization. Second, C, CC, and CCCL embedded NPI were cultured for 24 h with supplemented HAM’S F10 culture media to ensure no excess crosslinker remained in the matrices that could cause a negative reaction upon transplantation. These matrices were then transplanted subcutaneously in naïve immunoincompetent B6.Rag−/− mice. Pre-transplantation, at 4 d, and then weekly up to 28 d these grafts were retrieved and assessed for gross morphology, evidence of vascularization and islet survival. For histological assessment, retrieved collagen-based matrices were fixed in Shandon Zinc Formal-Fixx (Thermo Fisher Scientific, 6764255) then embedded in paraffin and 5 μm sections were prepared. To assess vascularization, von Willebrand Factor (vWF) staining was utilized to visualize arterioles and capillaries and islet survival, and sections of the grafts were also stained for insulin, hemotoxylin and eosin. After rehydration, antigen retrieval for vWF was performed in sodium citrate buffer (pH 6.0). All immunohistochemical samples were blocked with 20% normal goat serum for 20 min (NGS, Jackson ImmunoResearch Laboratories Inc., 0005-000-121). Slides were also stained with a guinea pig anti-insulin antibody (Dako, A564) diluted at 1:1000; a mouse anti-glucagon antibody (Sigma G2654) or a rabbit anti-vWF antibody (abcam, ab6994) diluted at 1:500. Slides were visualized with an Axioscope II microscope equipped with an AxioCam MRC and analyzed with Axiovision 4.6 software (Carl Zeiss, Gottingen, Germany). To quantify the degree of vascularization five images of vWF staining were taken from each of two retrieved matrices and the images were combined for particle analysis in ImageJ (National Institutes of Health). Particles were counted if they were between 50 and 10000 µm2 in size, with a circularity greater than 0.2. Each particle was confirmed to be a capillary visually and excluded by hand if mistakenly included by the software.
Statistical analysis
All statistics were performed using the Kruskal-Wallis 1-way analysis of variance with the Šidák correction; the level of significance was considered to be α = 0.05. All statistical analyses were performed with STATA 11 (StataCorp LP). Results are presented as mean ± SEM.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This study is supported by the Juvenile Diabetes Research Foundation (Grant #41-2009-771) and the Canadian Institutes of Health Research (Grant #MOP 119500). Ellis CE is a recipient of an Alberta Innovates Health Solutions Studentship. We would like to thank the Clinical Islet Laboratory (University of Alberta and Alberta Health Services) and Alberta Diabetes Institute IsletCore (University of Alberta) for providing human islet preparations; Dr Kunimasa Suzuki (Alberta Diabetes Institute Molecular Biology Core, University of Alberta) for assistance with the Meso Scale Discovery Assays; the University of Alberta Swine Research Centre for the neonatal piglets; the Alberta Diabetes Institute Histology Core including Sheena Maxwell for assistance with histology; and Deb Dixon for assistance with isolating the NPIs. The funders had no role in study design, data collection and analysis, decision to publish or publication of manuscript. Portions of this manuscript have been previously presented at the 13th World Congress of IPITA in Prague, Czech Republic (June 2011).
Glossary
Abbreviations:
- BSA
Bovine Serum Albumin
- CC
collagen and chitosan
- CCC
collagen, chitosan, and chondroitin
- CCCL
collagen, chitosan, chondroitin and laminin
- C
Collagen matrix
- EDC
1-ethyl-3-(3-dimethylaminopropyl) carbodiimide
- FBS
Fetal Bovine Serum
- HBSS
Hank’s Balanced Salt Solution
- IBMX
isobutylmethylxanthine
- NHS
N-hydroxysuccinimide
- NPI
neonatal porcine islets
- RPMI
Roswell Park Memorial Institute medium
- SDF-1
Stromal cell-derived factor-1
- VEGF
vascular endothelial growth factor
- vWF
von Willebrand factor
Footnotes
Previously published online: www.landesbioscience.com/journals/islets/article/27175
References
- 1.Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, Secchi A, Brendel MD, Berney T, Brennan DC, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006;355:1318–30. doi: 10.1056/NEJMoa061267. [DOI] [PubMed] [Google Scholar]
- 2.Ryan EA, Lakey JR, Rajotte RV, Korbutt GS, Kin T, Imes S, Rabinovitch A, Elliott JF, Bigam D, Kneteman NM, et al. Clinical outcomes and insulin secretion after islet transplantation with the Edmonton protocol. Diabetes. 2001;50:710–9. doi: 10.2337/diabetes.50.4.710. [DOI] [PubMed] [Google Scholar]
- 3.Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–8. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 4.Street CN, Lakey JRT, Shapiro AMJ, Imes S, Rajotte RV, Ryan EA, Lyon JG, Kin T, Avila J, Tsujimura T, et al. Islet graft assessment in the Edmonton Protocol: implications for predicting long-term clinical outcome. Diabetes. 2004;53:3107–14. doi: 10.2337/diabetes.53.12.3107. [DOI] [PubMed] [Google Scholar]
- 5.Villiger P, Ryan EA, Owen R, O’Kelly K, Oberholzer J, Al Saif F, Kin T, Wang H, Larsen I, Blitz SL, et al. Prevention of bleeding after islet transplantation: lessons learned from a multivariate analysis of 132 cases at a single institution. Am J Transplant. 2005;5:2992–8. doi: 10.1111/j.1600-6143.2005.01108.x. [DOI] [PubMed] [Google Scholar]
- 6.Bhargava R, Senior PA, Ackerman TE, Ryan EA, Paty BW, Lakey JR, Shapiro AM. Prevalence of hepatic steatosis after islet transplantation and its relation to graft function. Diabetes. 2004;53:1311–7. doi: 10.2337/diabetes.53.5.1311. [DOI] [PubMed] [Google Scholar]
- 7.Markmann JF, Rosen M, Siegelman ES, Soulen MC, Deng S, Barker CF, Naji A. Magnetic resonance-defined periportal steatosis following intraportal islet transplantation: a functional footprint of islet graft survival? Diabetes. 2003;52:1591–4. doi: 10.2337/diabetes.52.7.1591. [DOI] [PubMed] [Google Scholar]
- 8.Vincenti F, Larsen C, Durrbach A, Wekerle T, Nashan B, Blancho G, Lang P, Grinyo J, Halloran PF, Solez K, et al. Belatacept Study Group Costimulation blockade with belatacept in renal transplantation. N Engl J Med. 2005;353:770–81. doi: 10.1056/NEJMoa050085. [DOI] [PubMed] [Google Scholar]
- 9.Robertson RP. Islet transplantation as a treatment for diabetes - a work in progress. N Engl J Med. 2004;350:694–705. doi: 10.1056/NEJMra032425. [DOI] [PubMed] [Google Scholar]
- 10.Deng C, Zhang P, Li F, Griffith M, Ruel M, Suuronen EJ. An injectable collagen-chitosan hydrogel for heart repair. Can J Cardiol. 2008;24(suppl E):Abstract #911. [Google Scholar]
- 11.Suuronen EJ, Veinot JP, Wong S, Kapila V, Price J, Griffith M, Mesana TG, Ruel M. Tissue-engineered injectable collagen-based matrices for improved cell delivery and vascularization of ischemic tissue using CD133+ progenitors expanded from the peripheral blood. Circulation. 2006;114(Suppl):I138–44. doi: 10.1161/CIRCULATIONAHA.105.001081. [DOI] [PubMed] [Google Scholar]
- 12.Balamurugan AN, Gu Y, Tabata Y, Miyamoto M, Cui W, Hori H, Satake A, Nagata N, Wang W, Inoue K. Bioartificial pancreas transplantation at prevascularized intermuscular space: effect of angiogenesis induction on islet survival. Pancreas. 2003;26:279–85. doi: 10.1097/00006676-200304000-00012. [DOI] [PubMed] [Google Scholar]
- 13.De Vos P, Hillebrands JL, De Haan BJ, Strubbe JH, Van Schilfgaarde R. Efficacy of a prevascularized expanded polytetrafluoroethylene solid support system as a transplantation site for pancreatic islets. Transplantation. 1997;63:824–30. doi: 10.1097/00007890-199703270-00006. [DOI] [PubMed] [Google Scholar]
- 14.Hou YT, Ijima H, Takei T, Kawakami K. Growth factor/heparin-immobilized collagen gel system enhances viability of transplanted hepatocytes and induces angiogenesis. J Biosci Bioeng. 2011;112:265–72. doi: 10.1016/j.jbiosc.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 15.Madhavan K, Belchenko D, Motta A, Tan W, Tan W. Evaluation of composition and crosslinking effects on collagen-based composite constructs. Acta Biomater. 2010;6:1413–22. doi: 10.1016/j.actbio.2009.09.028. [DOI] [PubMed] [Google Scholar]
- 16.Liang WH, Kienitz BL, Penick KJ, Welter JF, Zawodzinski TA, Baskaran H. Concentrated collagen-chondroitin sulfate scaffolds for tissue engineering applications. J Biomed Mater Res A. 2010;94:1050–60. doi: 10.1002/jbm.a.32774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osborne CS, Barbenel JC, Smith D, Savakis M, Grant MH. Investigation into the tensile properties of collagen/chondroitin-6-sulphate gels: the effect of crosslinking agents and diamines. Med Biol Eng Comput. 1998;36:129–34. doi: 10.1007/BF02522870. [DOI] [PubMed] [Google Scholar]
- 18.Jalili RB, Moeen Rezakhanlou A, Hosseini-Tabatabaei A, Ao Z, Warnock GL, Ghahary A. Fibroblast populated collagen matrix promotes islet survival and reduces the number of islets required for diabetes reversal. J Cell Physiol. 2011;226:1813–9. doi: 10.1002/jcp.22515. [DOI] [PubMed] [Google Scholar]
- 19.Hosseini-Tabatabaei A, Jalili RB, Hartwell R, Salimi S, Kilani RT, Ghahary A. Embedding islet in a liquid scaffold increases islet viability and function. Can J Diabetes. 2013;37:27–35. doi: 10.1016/j.jcjd.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 20.Suuronen EJ, Nakamura M, Watsky MA, Stys PK, Müller LJ, Munger R, Shinozaki N, Griffith M. Innervated human corneal equivalents as in vitro models for nerve-target cell interactions. FASEB J. 2004;18:170–2. doi: 10.1096/fj.03-0043fje. [DOI] [PubMed] [Google Scholar]
- 21.Edamura K, Nasu K, Iwami Y, Ogawa H, Sasaki N, Ohgawara H. Effect of adhesion or collagen molecules on cell attachment, insulin secretion, and glucose responsiveness in the cultured adult porcine endocrine pancreas: a preliminary study. Cell Transplant. 2003;12:439–46. doi: 10.3727/000000003108746867. [DOI] [PubMed] [Google Scholar]
- 22.Korbutt GS, Elliott JF, Ao Z, Smith DK, Warnock GL, Rajotte RV. Large scale isolation, growth, and function of porcine neonatal islet cells. J Clin Invest. 1996;97:2119–29. doi: 10.1172/JCI118649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cardona K, Korbutt GS, Milas Z, Lyon J, Cano J, Jiang W, Bello-Laborn H, Hacquoil B, Strobert E, Gangappa S, et al. Long-term survival of neonatal porcine islets in nonhuman primates by targeting costimulation pathways. Nat Med. 2006;12:304–6. doi: 10.1038/nm1375. [DOI] [PubMed] [Google Scholar]
- 24.Zhou YF, Liu WZ. Laticiferous canal formation in fruits of Decaisnea fargesii: a programmed cell death process? Protoplasma. 2011;248:683–94. doi: 10.1007/s00709-010-0229-2. [DOI] [PubMed] [Google Scholar]
- 25.Kuraitis D, Zhang P, Zhang Y, Padavan DT, McEwan K, Sofrenovic T, McKee D, Zhang J, Griffith M, Cao X, et al. A stromal cell-derived factor-1 releasing matrix enhances the progenitor cell response and blood vessel growth in ischaemic skeletal muscle. Eur Cell Mater. 2011;22:109–23. doi: 10.22203/ecm.v022a09. [DOI] [PubMed] [Google Scholar]
- 26.McEwan K, Padavan DT, Deng C, Vulesevic B, Kuraitis D, Korbutt GS, Suuronen EJ. Tunable collagen hydrogels are modified by the therapeutic agents they are designed to deliver. J Biomater Sci Polym Ed. 2011;23:1467–83. doi: 10.1163/092050611X584397. [DOI] [PubMed] [Google Scholar]
- 27.Duffy GP, McFadden TM, Byrne EM, Gill SL, Farrell E, O’Brien FJ. Towards in vitro vascularisation of collagen-GAG scaffolds. Eur Cell Mater. 2011;21:15–30. doi: 10.22203/ecm.v021a02. [DOI] [PubMed] [Google Scholar]
- 28.Ito T, Itakura S, Todorov I, Rawson J, Asari S, Shintaku J, Nair I, Ferreri K, Kandeel F, Mullen Y. Mesenchymal stem cell and islet co-transplantation promotes graft revascularization and function. Transplantation. 2010;89:1438–45. doi: 10.1097/TP.0b013e3181db09c4. [DOI] [PubMed] [Google Scholar]
- 29.Ding Y, Xu D, Feng G, Bushell A, Muschel RJ, Wood KJH. Mesenchymal stem cells prevent the rejection of fully allogenic islet grafts by the immunosuppressive activity of matrix metalloproteinase-2 and -9. Diabetes. 2009;58:1797–806. doi: 10.2337/db09-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cavallari G, Olivi E, Bianchi F, Neri F, Foroni L, Valente S, La Manna G, Nardo B, Stefoni S, Ventura C. Mesenchymal stem cells and islet cotransplantation in diabetic rats: improved islet graft revascularization and function by human adipose tissue-derived stem cells preconditioned with natural molecules. Cell Transplant. 2012;21:2771–81. doi: 10.3727/096368912X637046. [DOI] [PubMed] [Google Scholar]
- 31.Yeung TY, Seeberger KL, Kin T, Adesida A, Jomha N, Shapiro AMJ, Korbutt GS. Human mesenchymal stem cells protect human islets from pro-inflammatory cytokines. PLoS One. 2012;7:e38189. doi: 10.1371/journal.pone.0038189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pileggi A, Molano RD, Ricordi C, Zahr E, Collins J, Valdes R, Inverardi L. Reversal of diabetes by pancreatic islet transplantation into a subcutaneous, neovascularized device. Transplantation. 2006;81:1318–24. doi: 10.1097/01.tp.0000203858.41105.88. [DOI] [PubMed] [Google Scholar]
- 33.Sakurai T, Satake A, Sumi S, Inoue K, Nagata N, Tabata Y, Miyakoshi J. The efficient prevascularization induced by fibroblast growth factor 2 with a collagen-coated device improves the cell survival of a bioartificial pancreas. Pancreas. 2004;28:e70–9. doi: 10.1097/00006676-200404000-00028. [DOI] [PubMed] [Google Scholar]
- 34.Stiegler P, Matzi V, Pierer E, Hauser O, Schaffellner S, Renner H, Greilberger J, Aigner R, Maier A, Lackner C, et al. Creation of a prevascularized site for cell transplantation in rats. Xenotransplantation. 2010;17:379–90. doi: 10.1111/j.1399-3089.2010.00606.x. [DOI] [PubMed] [Google Scholar]
- 35.Wang W, Gu Y, Hori H, Sakurai T, Hiura A, Sumi S, Tabata Y, Inoue K. Subcutaneous transplantation of macroencapsulated porcine pancreatic endocrine cells normalizes hyperglycemia in diabetic mice. Transplantation. 2003;76:290–6. doi: 10.1097/01.TP.0000073613.25658.4D. [DOI] [PubMed] [Google Scholar]
- 36.Kin T, Korbutt GS, Rajotte RV. Survival and metabolic function of syngeneic rat islet grafts transplanted in the omental pouch. Am J Transplant. 2003;3:281–5. doi: 10.1034/j.1600-6143.2003.00049.x. [DOI] [PubMed] [Google Scholar]
- 37.Ao Z, Matayoshi K, Lakey JRT, Rajotte RV, Warnock GL. Survival and function of purified islets in the omental pouch site of outbred dogs. Transplantation. 1993;56:524–9. doi: 10.1097/00007890-199309000-00007. [DOI] [PubMed] [Google Scholar]
- 38.Yasunami Y, Lacy PE, Finke EH. A new site for islet transplantation--a peritoneal-omental pouch. Transplantation. 1983;36:181–2. doi: 10.1097/00007890-198308000-00014. [DOI] [PubMed] [Google Scholar]