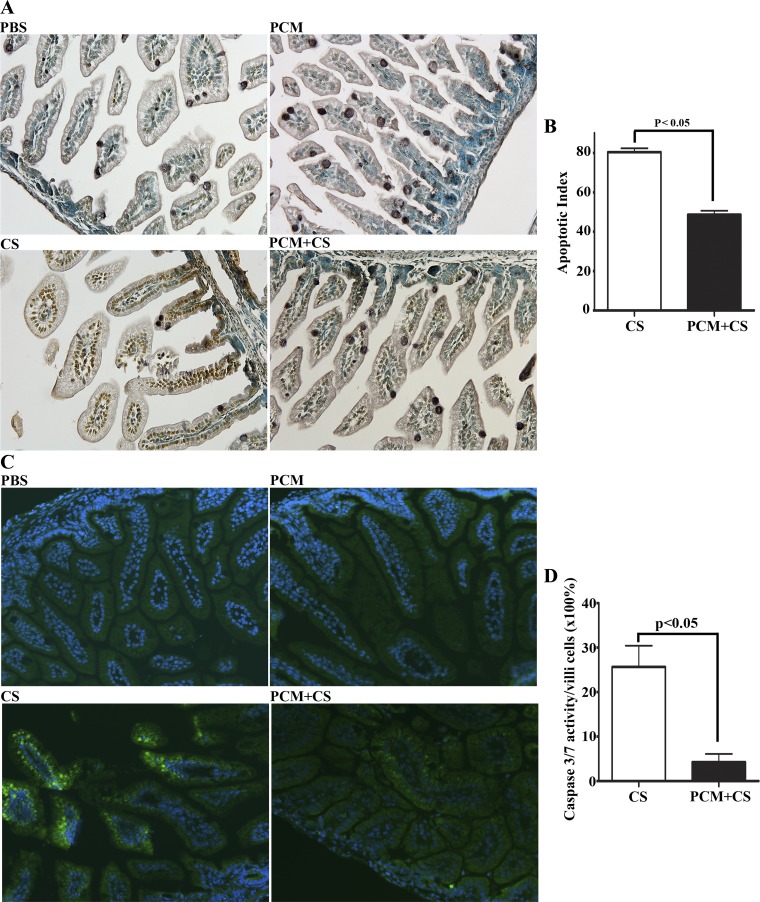
Fig. 4.
PCM attenuates apoptotic enterocyte cell death in C. sakazaii-infected ileum. Apoptotic cells in the paraffin-embedded ileum sections from each group were used in an immunoperoxidase-based TUNEL assay (A and B) and an activated caspase enzyme detection fluorescent labeled inhibitor of caspases (FLICA) caspase assay (C and D). A: images collected via a Nikon PL APO ×20 objective (n = 10 mice in each group). No apoptotic cells could be detected in ileal sections of mice treated with PBS or with PCM alone. C. sakasakii infection triggered severe apoptosis of epithelial cells in all examined ileal sections that was attenuated by PCM treatment of mice prior to infection. B: 10 villi and crypts of each section from PCM plus C. sakasakii and C. sakasakii-alone groups were observed under a light microscope, and the ratio of the number of TUNEL-positive cells to the total number of cells in the 10 villi and crypts was assigned as the apoptotic index (n = 10 mice in each group, means ± SE, Student's t-test: P < 0.05). C: active caspase-3 and -7 enzymes in paraffin-embedded ileal sections were labeled by a green fluorescent inhibitor probe FAM-DEVD-FMK (green). Nuclei were indicated with DAPI staining (blue), ×200 magnification. Treatment conditions are shown at bottom left of each image. D: 10 villi of each section from PCM plus C. sakasakii and C. sakasakii-alone groups were observed under a light microscope, and the ratio of the number of activated caspase 3&7 (green) cells to the total number of cells in the 10 villi (×100%) was calculated and graphed (n = 5 mice in each group, means ± SE, Student's t-test: P < 0.05).