Fig. 3.
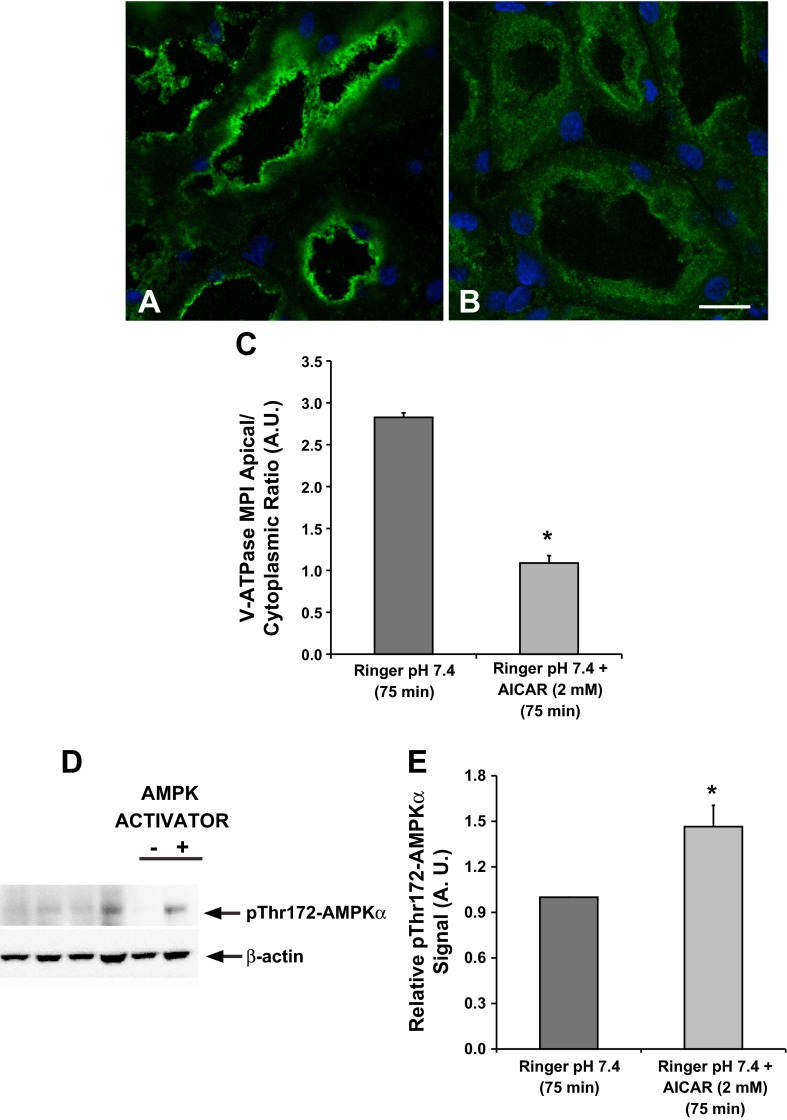
The AMP-activated protein kinase (AMPK) activator 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR) prevents V-ATPase apical accumulation in the proximal tubule S3 segment. Confocal images illustrate the cellular distribution changes of the V-ATPase E subunit in the S3 segment of kidney slices treated with Ringer buffer in the absence/presence of the AMPK activator AICAR (2 mM; 75 min). A: incubation of kidney slices in buffer alone for 75 min induced apical V-ATPase accumulation in S3 proximal tubules. B: addition of AICAR induced more diffuse cytosolic V-ATPase labeling in S3 segments compared with buffer alone. C: quantification of the mean (±SE) V-ATPase-associated MPI apical-to-cytoplasmic ratio from three separate experiments confirmed a significant cytoplasmic redistribution of the V-ATPase in the presence of AICAR (*P < 0.05 vs. Ringer, pH 7.4, 75 min. Scale bar = 10 μm). D: representative immunoblot of rat kidney lysates showing AMPK activity (pThr172-AMPKα; top) with β-actin as loading control (bottom) following treatment of kidney slices with Ringer buffer in the absence or presence of the AMPK activator AICAR (2 mM; 75 min). E: AICAR treatment induced a significant upregulation of phosphorylated pThr172-AMPKα compared with untreated cells. Values are relative means (±SE) of pThr172-AMPKα signal for 3 separate slices from 3 different animals normalized to β-actin (*P < 0.05 vs. Ringer, pH 7.4, 75 min).