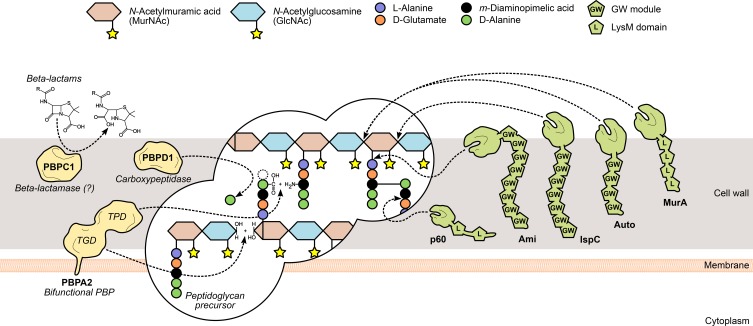
Figure 2.
L. monocytogenes peptidoglycan metabolism and the surface proteins involved in its assembly and turnover. The peptidoglycan sacculus is polymerized with cytoplasmic precursors with the help of penicillin-binding proteins (PBPs, yellow). High-molecular-weight PBPs, such as PBPA2, contain transglycosylase (TGD) and transpeptidase domains (TPD) that catalyze, respectively, glycan chain elongation and stem peptide bridging between adjacent chains. Other PBPs include the low-molecular-mass carboxypeptidases, which cleave the terminal D-alanyl-D-alanine stem peptide bond (e.g., PBPD1), and beta-lactamases, which degrade PBP-inhibiting antibiotics to promote bacterial survival (e.g., PBPC1). On the other hand, the degradation of mature peptidoglycan, during bacterial elongation/division or autolysis, is mediated by autolysins (green), a family of surface hydrolases that can cleave the peptidoglycan at different sites: within the glycan chain (N-acetylglucosaminidases or N-acetylmuramidases) or the stem peptide (endo- and carboxypeptidases), or between both (N-acetylmuramoyl-L-alanine amidases). Interestingly, autolysins commonly associate non-covalently with the bacterial surface via cell wall-binding repeats, such as the GW modules in Ami, Auto and IspC, or the LysM repeats in MurA and p60.