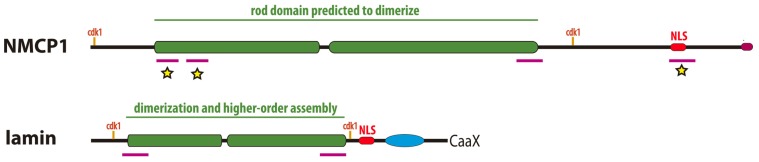
FIGURE 3.
Comparison of the structure of plant NMCP proteins and metazoan lamins. Both proteins have a similar tripartite structure with a central coiled–coil domain (green boxes) flanked by a short head and a long tail domains. The rod domain which is responsible for dimerization and higher order assembly in lamins, presents highly conserved regions at both ends (magenta bars) involved in head to tail association of dimers in the case of lamins. The rod domain is flanked by conserved cdk1 phosphorylation sites in both cases. In the tail domain both have a NLS (red boxes) and a conserved C-terminus (magenta box in NMCP1 and CAAX box in lamins). NMCPs lack the Ig fold for partner protein binding typical of lamins (blue oval). The conserved regions marked with a yellow star are involved in NMCP1 association to the nuclear periphery (Kimura et al., 2014).