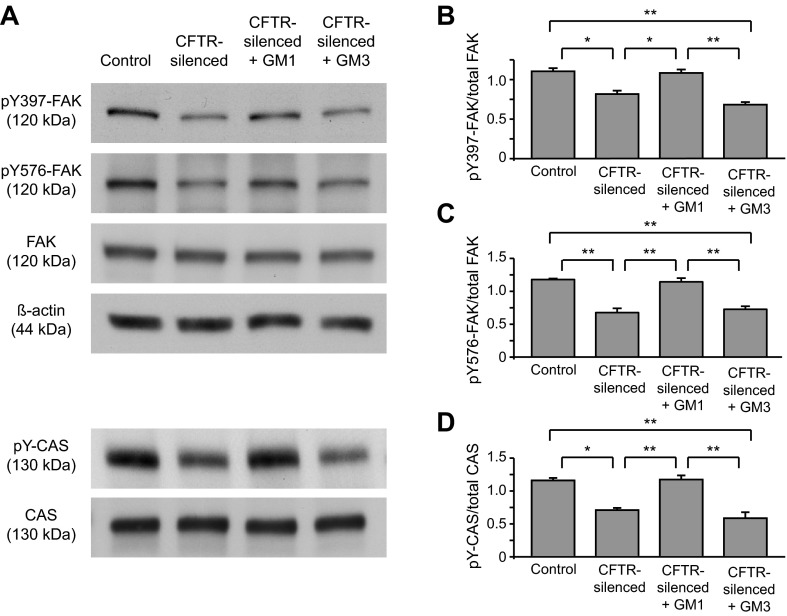
Fig. 6.
Focal adhesion kinase (FAK) and Crk-associated substrate (CAS) phosphorylation is reduced in CFTR silencing and restored by GM1. Control and CFTR-silenced cells were cultured in serum-free medium for 2 days. In separate samples, CFTR-silenced cells were cultured over this time with 5 μM GM1 or GM3. Cell lysates were collected and immunoblotted for total FAK, Y397, and Y576 phospho-FAK, β-actin, and total CAS. Equal protein quantities (20 μg) were loaded per lane. Phosphorylated CAS (pY-CAS) was detected by immunoprecipitating with a phospho-tyrosine antibody and probing with an antibody against CAS. A: representative immunoblots. B–D: quantitation of pY397-FAK (B), pY576-FAK (C), and pY-CAS (D). Signals on immunoblots were quantified by image analysis. Ratios of pY-FAK/total FAK and pY-CAS/total CAS were calculated for each replicate sample. Values were normalized by dividing the phospho-/total ratios for each replicate by (the sum of phospho-/total protein ratios for controls, CFTR-silenced, and CFTR-silenced + GM1 from the same blot pair)/3. Results are means ± SE of ≥3 replicates per condition. *P < 0.01 and **P < 0.001, significant differences between indicated groups as calculated using one-way ANOVA with a Bonferroni post test.