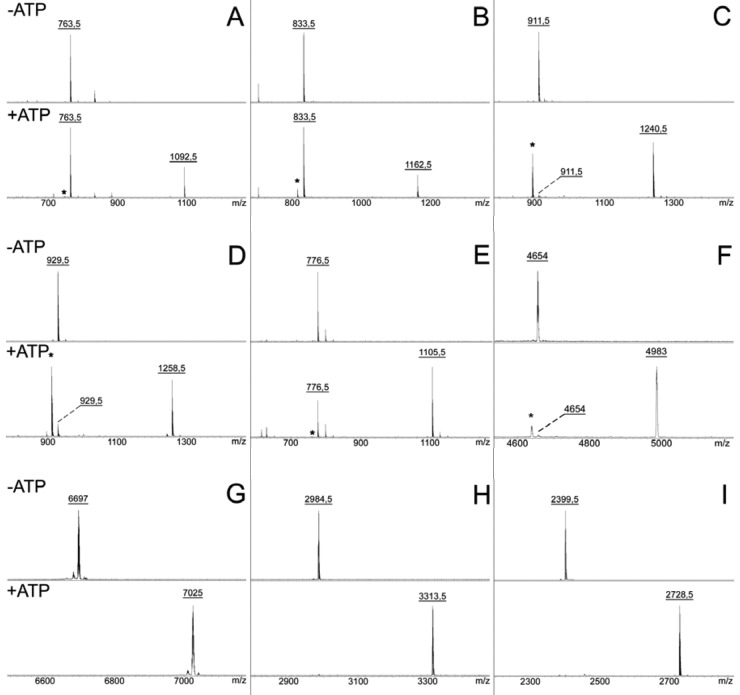
FIG 2 .
In vitro
adenylation of predicted MccA peptides by cognate MccB homologs. Chemically synthesized peptides corresponding to 7-amino-acid-long MccA products of E. coli (A), B. washoensis Sb944nv (B), H. pylori plasmid HPP12 (C), L. johnsonii NCC 533 (D), S. thermophilus LMD-9 (E), Y. pseudotuberculosis PB1/+ (F), full-length Synechococcus sp. CC9605 peptide (G), and peptides corresponding to the last 25 (H) and 20 (I) amino acids of Synechococcus sp. CC9605 peptide (Table 1) were combined with the corresponding recombinant MccB homologs in the absence (top) and the presence (bottom) of ATP. Reaction products were analyzed by MALDI MS. Only relevant parts of the spectra are shown. Asterisks indicate peaks corresponding to adenylation reaction intermediates containing a terminal succinimide (Fig. 1A).