FIG 6 .
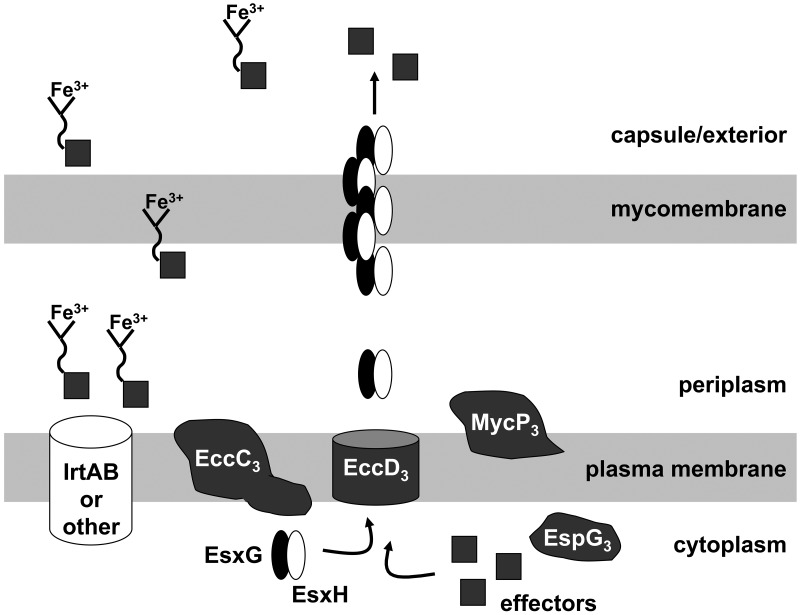
One model for Esx-3 function in which EsxG and EsxH are both substrates and chaperones or structural components of the secretion apparatus. IrtA and IrtB are components of a mycobactin transporter system (63–65). We hypothesize that at least one of the functions of EsxG and EsxH is to ferry effectors of iron-loaded mycobactin uptake within or across the mycobacterial cell wall. In the absence of an Esx-3 core component, such as EccD3, we do not detect EsxG or EsxH in culture supernatants. We hypothesize that the proteins are not exported across the cytoplasmic membrane under this condition and therefore are completely unable to contribute to iron acquisition. In contrast, although supernatants from fxbA ΔmycP3ms cultures do not contain detectable EsxG or EsxH, the strain itself retains intermediate growth under low-iron conditions. We suggest the MycP3 protease may be an accessory Esx-3 component that is required either for a stable EsxG-EsxH structure or for optimal chaperone activity. Thus, in the absence of this accessory factor, EsxG and EsxH may still traverse the cytoplasmic membrane to the periplasm but be only partially functional.