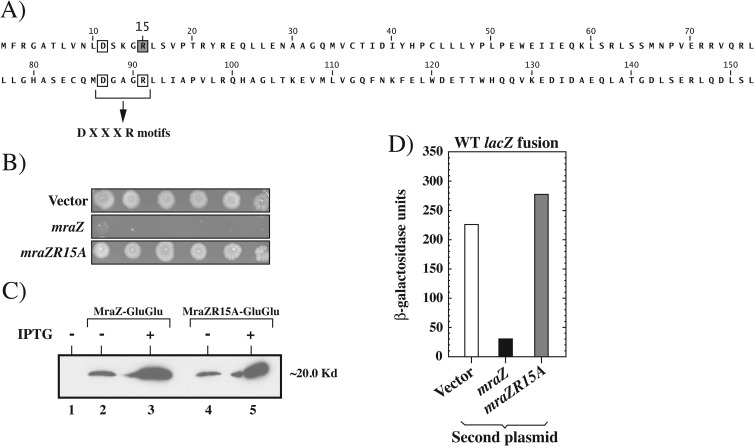
FIG 5.
Characterization of the MraZR15A DNA-binding mutant. (A) Sequence of the MraZ protein from E. coli, representing the two copies of the UPF0040 fold (19), including amino acids 1 to 76 (top row) and 77 to 152 (bottom row). The two DXXXR motifs are highlighted; they correspond to amino acids D11 to R15 and D87 to R91. Arginine R15 (shaded square) in the first motif was mutated to alanine in the MraZR15A mutant. (B) Spot dilution assay of WT MG1655 containing the pDSW208 vector, pDSW208-mraZ, or pDSW208-mraZR15A on LB plates containing 1 mM IPTG to fully induce gene expression. (C) Levels of MraZR15A are similar to those of MraZ. WT MG1655 cells containing the pDSW208 vector (lane 1), pDSW208-mraZ-gluglu (lanes 2 and 3), or pDSW208-mraZR15A-gluglu (lanes 4 and 5) were grown in LB to an OD600 of ∼0.2. Cells expressing mraZ on the plasmid were either left uninduced (lanes 2 and 4) or induced (lanes 3 and 5) with 1 mM IPTG and grown for an additional 2 h. Aliquots were normalized for total protein and subjected to SDS-PAGE. Blots were detected with anti-GluGlu antibodies. The location of 20 Kd is where the 20-kDa molecular size marker runs. (D) β-Galactosidase assays comparing the effects of pDSW208 (vector), (pDSW208/mraZ), and (pDSW208/mraZR15A) on the mraZ-lacZ reporter after induction with 50 μM IPTG for 30 min. β-Galactosidase activity is expressed in μmol/min/mg protein. One representative experiment is shown.