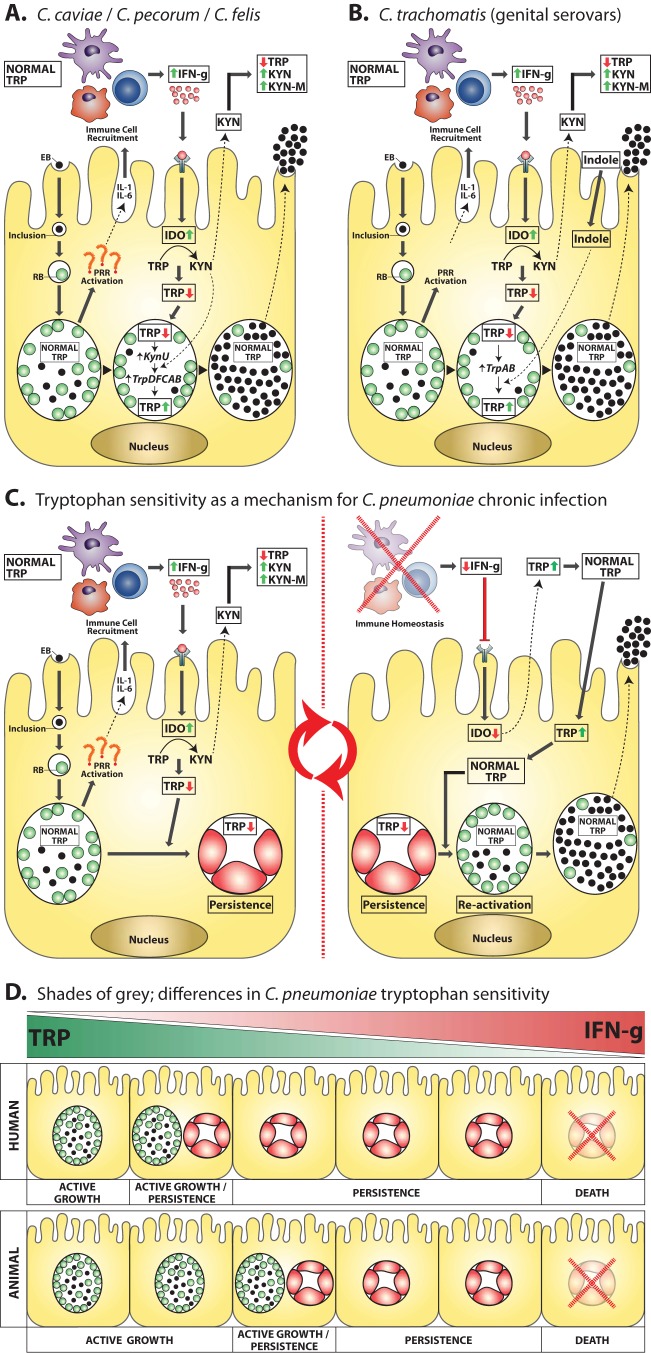
FIG 1.
The central role of tryptophan in chlamydial biology. Chlamydial elementary bodies attach to and enter a host epithelial cell, an inclusion is formed, and the elementary body (EB) converts to its replicative form, the reticulate body (RB). RBs undergo replication; however, during infection, PRRs are activated, resulting in secretion of proinflammatory cytokines from the host, which in turn recruits immune cells. Development of an immune response results in host cells being exposed to gamma interferon. IDO is activated, and tryptophan pools are depleted. (A) The species C. caviae, C. pecorum, and C. felis obtain kynurenine (the product of IDO activity) from the host cell and use it to recycle tryptophan via tryptophan biosynthesis genes (kynU and trpD, -F, -C, -A, and -B), meaning that these species are able to actively replicate during IDO activity and host tryptophan depletion. This tryptophan biosynthesis process is regulated by a tryptophan repressor and thus is initiated only when inclusion tryptophan levels become depleted. (B) The urogenital strains of C. trachomatis are believed to respond to IDO-tryptophan depletion by using indole (thought to be supplied by the vaginal microflora) as a precursor for tryptophan synthesis. Tryptophan synthesis is achieved through the tryptophan biosynthesis genes trpA and -B. As with the above-mentioned species, C. trachomatis tryptophan biosynthesis is also regulated via a tryptophan repressor that allows biosynthesis to occur only once inclusion tryptophan levels become depleted. Interestingly, the ocular C. trachomatis strains do not possess the tryptophan biosynthesis genes and are associated with more-chronic disease outcomes. (C) The key role of tryptophan in C. pneumoniae chronic infection. In stark contrast to the above-mentioned species, C. pneumoniae does not contain any tryptophan biosynthesis genes. As a result, tryptophan depletion results in conversion of an active replicating inclusion to a persistent inclusion (a viable but nonreplicating, static phase). In this state, C. pneumoniae becomes refractory to immune insult, allowing the organism to hide in the host cell until the immune response subsides. As the immune response abates, IFN-γ becomes depleted, IDO is no longer induced, local tryptophan pools become recharged, and tryptophan levels eventually return to normal. At this point, C. pneumoniae reverts to an actively replicating inclusion, resulting in the release of infectious progeny ready to reinitiate the chronic infection cycle. (D) Recent studies have found that human and animal (ancestral) strains behave very differently in the face of IFN-γ insult and tryptophan depletion. This finding suggests that the human strains have evolved to become more sensitive to IFN-γ, allowing them to enter a persistent phase at the first signs of an immune response. As a result, they have been able to develop a chronic infection cycle that has enabled them to become, arguably, one of the most successful human pathogens. EB, elementary body; RB, reticulate body; IFN-g, gamma interferon; IL, interleukin; PRR, pattern recognition receptors; IDO, indoleamine 2,3-dioxygenase; TRP, tryptophan; KYN, kynurenine; KYN-M, kynurenine metabolites.