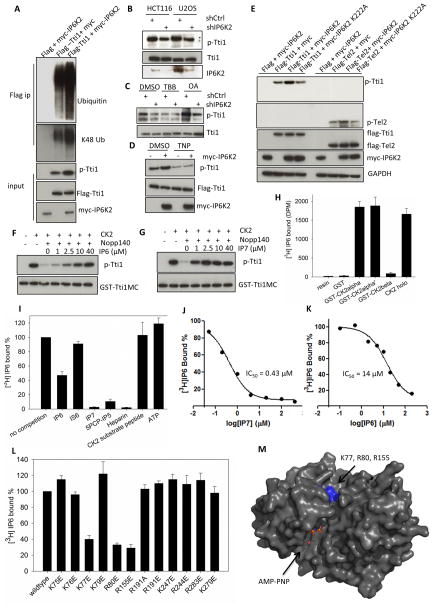
Figure 2. IP7 binds to CK2 and enhances CK2-mediated phosphorylation of Tti1/Tel2.
(A) Coexpression of IP6K2 increases the phosphorylation (S828) and ubiqutination of Tti1. Cells were harvested 28 h after transfection. The amount of Tti1 plasmids used for transfection was adjusted to achieve equal expression levels. (B) Lentiviral shRNA knockdown of IP6K2 diminishes endogenous Tti1 phosphorylation. (C) Effect of CK2 inhibitor TBB (20 μM, 4 h) and protein phosphatase inhibitor okadaic acid (OA) (50 nM, 1 h) on Tti1 phosphorylation. U2OS cells were transfected with the indicated lentiviral shRNA constructs and selected with puromycin (1 μg/ml) prior to drug treatment. (D) Effect of TNP treatment (10 μM, 1.5 h) on the phosphorylation of Tti1, with/without IP6K2 co-expression. (E) Co-expression of IP6K2 wildtype, but not the K222A mutant, increases phospho-Tti1 (S828) and phospho-Tel2 (S487/S491). (F–G) Concentration-dependent effects of IP6 (F) and IP7 (G) on reversing hNopp140 (500 nM) inhibition of GST-Tti1MC phosphorylation by CK2. Reaction conditions were: 20 mM MgCl2 (pH 7.5), 50 mM KCl, 10 mM MgCl2, 200 μM ATP, 30 °C, 15 min. (H) [3H]IP6 binding to various CK2 preparations. Twenty μg purified recombinant CK2 proteins on glutathione beads were incubated with 10 μl [3H]IP6 (65 μCi/ml) overnight at 4 °C. Controls included buffer or GST alone. (I–J) Concentration-dependent competition of [3H]IP6 binding to CK2α by unlabeled IP6 (I) and IP7 (J). (K) [3H]IP6 binding to CK2α in the presence of various competitors. The concentrations of small molecules used were: ATP (100 μM), the rest (25 μM). (L) [3H]IP6 binding to CK2α mutants. (M) Location of K77, R80 and R155 in the 3D structure of CK2α displayed in surface mode (PDB id: 2PVR). The ATP analog AMPPNP is located in the catalytic active site. Residues K77, R80 and R155 are highlighted in blue. The figure was generated by Pymol. see also Figure S2.