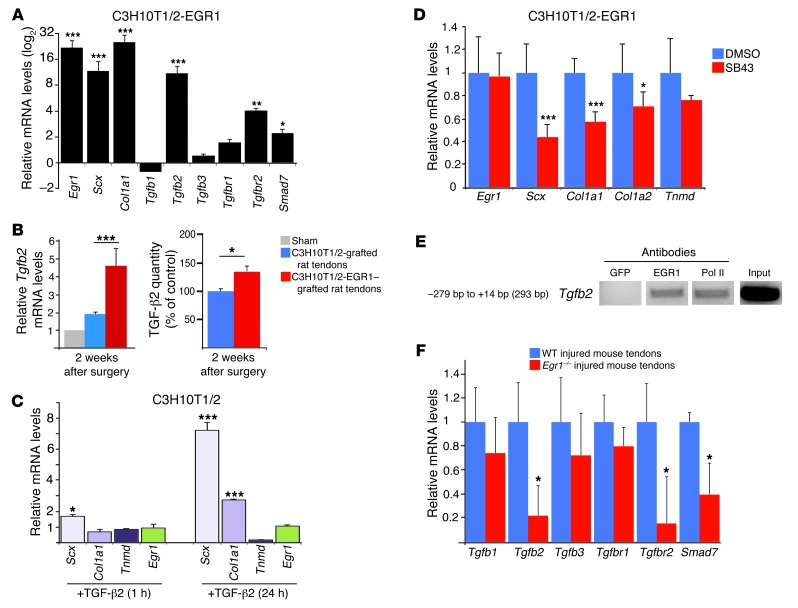
Figure 9. Link between Egr1 and TGF-β signaling pathway components during tendon cell differentiation in postnatal tendons and injured tendons.
(A) EGR1-producing C3H10T1/2 cells displayed increased mRNA expression levels of the TGF-β signaling pathway components, Tgfb2, Tgfbr2, and Smad7, compared with control C3H10T1/2 cells. mRNA levels for each gene in the control C3H10T1/2 cells were established at 1. (B) Tgfb2 mRNA levels and TGF-β2 quantity were determined in manipulated rat tendons grafted with C3H10T1/2-EGR1 versus control C3H10T1/2 cells 2 weeks postoperation. (C) Application of human recombinant TGF-β2 in C3H10T1/2 cells led to an increase in Scx and Col1a1 expression, while that of Egr1 and Tnmd was not induced 1 or 24 hours after TGF-β2 exposure. (D) Application of a specific TGF-β inhibitor SB43 on C3H10T1/2-EGR1 cells diminished the mRNA expression levels of Scx and Col1a1 genes. mRNA levels of C3H10T1/2-EGR1 cells treated with DMSO were normalized to 1. (E) ChIP assays were performed on tendons from postnatal mice with antibodies against EGR1. ChIP products were analyzed by PCR. Primers targeting a 293-bp fragment of the Tgfb2 promoter identified DNA regions immunoprecipitated by EGR1. (F) qRT-PCR analyses of TGF-β pathway components in Egr1–/– injured tendons versus WT injured tendons 1 week after injury. mRNA levels of injured tendons from Egr1–/– and WT mice were normalized to those of Gapdh in each experiment. For qRT-PCR analyses, the error bars represent SEM. *P < 0.05; **P < 0.01; ***P < 0.001.