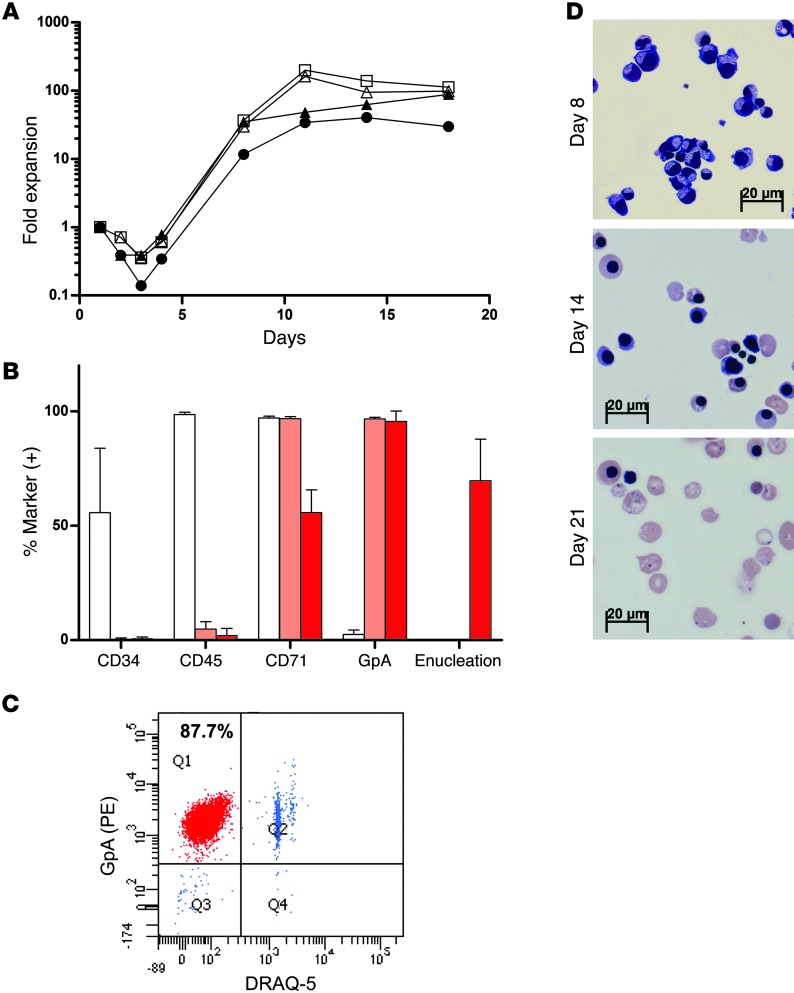
Figure 3. In vitro erythroid differentiation of BM CD34+ cells.
(A) Fold expansion from BM CD34+ cells grown under in vitro erythroid differentiation conditions over time. The growth curves from a representative experiment are shown. HD-mock, black triangles; HD-βAS3-FB transduced, black circles; SCD-mock, white triangles; SCD-βAS3-FB transduced, white squares. (B) Immunophenotypic analysis of CD34+ BM SCD–transduced samples during in vitro erythroid culture. Cells were analyzed by flow cytometry for expression of CD34, CD45, CD71, and GpA. Each bar represents the percentage of expression of the indicated surface marker at day 3 (white bars), day 14 (pink bars), and day 21 (red bars). Values shown are mean ± SD of 4 independent experiments. Percentage of enucleated rbc was assessed at day 21 (mean ± SD of 7 independent experiments) by staining with the DNA dye DRAQ5. (C) Flow cytometry analysis of erythroid culture to quantify enucleated rbc. Analysis was made by staining cells with DRAQ5 and antibody to human erythroid marker GpA. Enucleated erythrocytes are present in the left upper quadrant as DRAQ5-negative, GpA-positive cells. (D) Photomicrographs of cytocentrifuge preparations from cultures stained by May-Grunwald-Giemsa showing the progression of erythroid differentiation from erythroblast to normoblast at day 8 and 14 to a mostly uniform population of enucleated reticulocytes and erythrocytes at day 21.