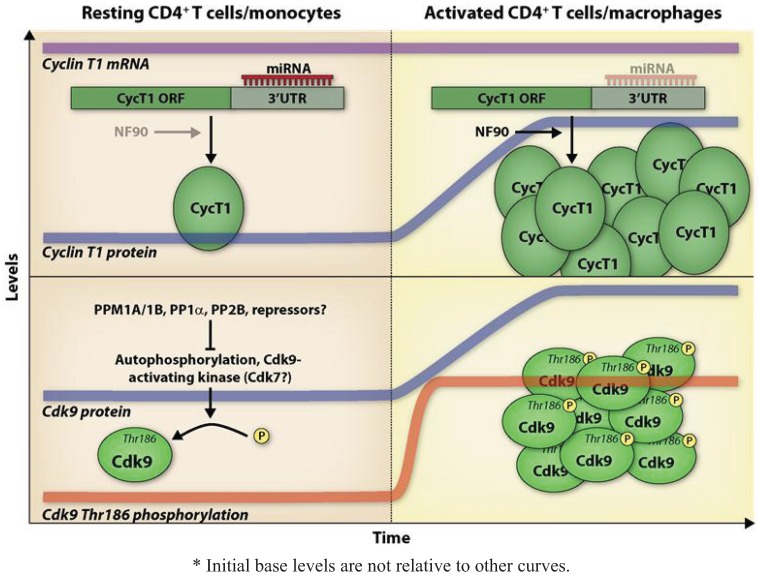
Figure 1.
Proposed model for P-TEFb regulation in resting CD4+ T cells/monocytes *. In resting CD4+ T cells and monocytes, Cyclin T1 protein levels are low, with protein translation being repressed via the action of Cyclin T1-targeting miRNAs. Additionally, it has been shown that the RNA-binding protein NF90, which acts to stimulate Cyclin T1 translation, is expressed at low levels in promonocytic cells. Following CD4+ T cell activation or monocyte differentiation, Cyclin T1 protein levels undergo a dramatic increase independently of any increase in Cyclin T1 mRNA levels; expression of Cyclin T1-targeting miRNAs is concomitantly decreased. Activation of promonocytic cells induces NF90 expression, which further contributes to increased Cyclin T1 translation. Cell activation also leads to Cdk9 protein induction and an increase in Cdk9 Thr186 phosphorylation, which occurs very rapidly in CD4+ T cells (depicted here), and at a more delayed rate during macrophage differentiation. This phosphorylation is thought to be mediated by autophosphorylation or a Cdk9-activating kinase. It is unclear how low levels of Thr186-phosphorylated Cdk9 are maintained in the resting cell, although it has been shown that Thr186 dephosphorylation can be mediated by PPM1A, PPM1B, PP1α, and PP2B and it is likely that these phosphatases or other repressors are involved.