Abstract
Aim:
The cholesterol-lowering drugs statins could enhance the activities of endothelial nitric oxide synthase (eNOS) and protect myocardium during ischemia and reperfusion. The aim of this study was to examine whether protein kinase A (PKA) was involved in statin-mediated eNOS phosphorylation and cardioprotection.
Methods:
6-Month-old Chinese minipigs (20–30 kg) underwent a 1.5-h occlusion and 3-h reperfusion of the left anterior descending coronary artery (LAD). In the sham group, the LAD was encircled by a suture but not occluded. Hemodynamic and cardiac function was monitored using a polygraph. Plasma activity of creatine kinase and the tissue activities of PKA and NOS were measured spectrophotometrically. p-CREB, eNOS and p-eNOS levels were detected using Western blotting. Sizes of the area at risk, the area of no-reflow and the area of necrosis were measured morphologically.
Results:
Pretreatment of the animals with simvastatin (SIM, 2 mg/kg, po) before reperfusion significantly decreased the plasma activity of creatine kinase, an index of myocardial necrosis, and reduced the no-reflow size (from 50.4%±2.4% to 36.1%±2.1%, P<0.01) and the infarct size (from 79.0%±2.7% to 64.1%±4.5%, P<0.01). SIM significantly increased the activities of PKA and constitutive NOS, and increased Ser133 p-CREB protein, Ser1179 p-eNOS, and Ser635 p-eNOS in ischemic myocardium. Intravenous infusion of the PKA inhibitor H-89 (1 μg·kg-1·min-1) partially abrogated the SIM-induced cardioprotection and eNOS phosphorylation. In contrast, intravenous infusion of the eNOS inhibitor L-NNA (10 mg·kg-1) completely abrogated the SIM-induced cardioprotection and eNOS phosphorylation during ischemia and reperfusion, but did not affect the activity of PKA.
Conclusion:
Pretreatment with a single dose of SIM 2.5 h before reperfusion attenuates myocardial no-reflow and infarction through increasing eNOS phosphorylation at Ser1179 and Ser635 that was partially mediated via the PKA signaling pathway.
Keywords: heart, ischemia-reperfusion injury, no-reflow, infarction, simvastatin, H-89, L-NNA, protein kinase A, CREB, endothelial NOS
Introduction
Timely reopening of the occluded coronary artery after acute myocardial infarction rescues the ischemic myocardium and reduces mortality. However, impaired regional perfusion and microvascular or endothelial dysfunction within the previously ischemic myocardium after revascularization therapy also produce the no-reflow phenomenon, which may lead to increased infarct size, contractile dysfunction, higher incidence of complications, and poor clinical outcome1,2,3,4. Prevention and treatment of the no-reflow phenomenon have been a worldwide challenge in this reperfusion times.
Statins are cholesterol-lowering drugs that inhibit 3-hydroxy-3-methylglutaryl coenzyme A reductase, a key enzyme in the cholesterol synthesis pathway. Previous studies have demonstrated that chronic and early pretreatment with statins can improve coronary circulation and reduce the sizes of no-reflow and infarct after ischemia-reperfusion injury by enhancing endothelial (e-)nitric oxide synthase (eNOS) activity through the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt) signaling cascade4,5,6,7,8,9,10.
According to the European Society of Cardiology (ESC) guidelines, the optimal revascularization time is within 3 h after the onset of acute myocardial infarction11. In almost all of the existing studies, statins were delivered several days before myocardial reperfusion. It is not clinically practical to pre-treat patients undergoing acute post-infarct percutaneous coronary intervention with high-dose statins several days before the procedure. In addition, recent studies have reported that the cyclic adenosine monophosphate (cAMP)/protein kinase A (PKA) pathway plays a role in cardioprotection during ischemic preconditioning and in the cardioprotection provided by Tongxinluo, a traditional Chinese medicine12,13,14, but it is unclear whether PKA is associated with the cardioprotective effects of statins. Therefore, in this study, we tested the hypothesis that acute pretreatment with single-dose statins before reperfusion exerts a cardioprotective effect against myocardial no-reflow and infarction by enhancing eNOS activity in a PKA-dependent manner.
Materials and methods
Animal experimental protocols
The animal experimental protocols and procedures were approved by the Care of Experimental Animals Committee of Fu Wai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, China. All animals received humane care in compliance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health, USA.
As described previously4,14, 6-month-old Chinese Minipigs weighing 20 to 30 kg were anesthetized with a mixture of 700 mg ketamine hydrochloride and 30 mg diazepam administered intramuscularly and were continuously infused with this mixture (2 mg/kg per hour) intravenously to maintain the anesthesia. Minipigs were assigned to 1 of 7 groups (n=7–8 in each group): control, simvastatin (SIM), SIM coadministered with H-89 (SIM+H-89), H-89, SIM coadministered with Nω-nitro-L-arginine (L-NNA; SIM+L-NNA), L-NNA, and sham group. All pigs except for the sham group underwent a 1.5-h occlusion and 3-h reperfusion of the left anterior descending coronary artery (LAD). The LAD of the sham animals was encircled by a suture but not occluded.
The control pigs underwent no intervention either before or after reperfusion. SIM (2 mg/kg, Merck & Co, USA) was gavaged 2.5 h before myocardial reperfusion; the SIM dosage was determined based on the loading dose (80 mg) before acute percutaneous coronary intervention and was converted to the pig dose according to body surface area15. H-89 (1.0 μg·kg-1·min-1, Alexis, USA), a PKA inhibitor, was intravenously and constantly infused throughout the procedure to inhibit PKA activity13. L-NNA (10 mg/kg, Aldrich, USA), an arginine derivative that can nonselectively and competitively inhibit NOS, was intravenously infused and maintained until the end of reperfusion to inhibit eNOS activity16. Although L-NNA inhibits both constitutive (cNOS) and inducible (iNOS), the effect of L-NNA inhibition on cNOS is 300 fold greater than that on iNOS, and this effect is rapidly reversible. Furthermore, the predominantly expressed isoform of cNOS in the myocardium is eNOS17. Therefore, we chose to use L-NNA as a selective inhibitor of eNOS in the present study.
Hemodynamic and cardiac function studies
Heart rate (HR) was monitored by surface limb lead electrocardiograph. A 6F pigtail catheter was inserted into the right femoral artery though an arterial sheath for real time measurements of mean arterial pressure (MAP), left ventricular end-diastolic pressure (LVEDP), and maximum and minimum rates of left ventricular pressure development (dp/dtmax and dp/dtmin, respectively). Hemodynamic data were recorded on a polygraph (Biopac Systems, MP-150, USA) at baseline, after 1.5 h of ischemia, and after 3 h of reperfusion and analyzed with Acqknowledge v3.8.1 software.
Analysis of myocardial area at risk (AAR), area of no-reflow (ANR), and area of necrosis (AN)
Myocardial AAR, ANR, and AN were measured according to previous methods4,14. In brief, the area of impaired perfusion was delineated by a bolus of 4% fluorescent thioflavin S (1 mL/kg, Sigma, USA) into the left atrium. Approximately 30 s later, the LAD was religated at the original site, and AAR was outlined by perfusion with a bolus of 2% Evans blue dye (1 mL/kg, Sigma, USA) into the left atrium. The heart was then excised, and the blood was washed out. In ice-cold saline solution, the extra-left ventricular tissue was removed, and the left ventricular tissue was transversely cut into six or seven slices made parallel to the atrioventricular groove. The AAR, or the area unstained by Evans blue, was traced and pictured in visible light. The ANR, or the area not perfused by thioflavin S, was photographed under ultraviolet light (365 nm). The area between the AAR and ANR was the area of reflow (AR). Then, tissue samples were collected from ANR, AR, and non-ischemic area (NA) on the reverse side of the traced slices and immediately placed in liquid nitrogen for the next examination. Finally, tissue slices were weighed and incubated in 1% triphenyltetrazolium chloride (TTC, pH 7.4) at 37 °C for 15 min to identify the AN. AAR was expressed as a percentage of the left ventricular mass (AAR/LV), and ANR and AN were expressed as percentages of the AAR (ANR/AAR and AN/AAR, respectively), with the mass of each area determined gravimetrically.
Determination of plasma creatine kinase (CK) activity
Plasma CK activity, an index of myocardial necrosis, was measured spectrophotometrically at baseline, after 1.5 h of ischemia, and after 3 h of reperfusion according to the manufacturer's instructions (Nanjing JianCheng Bioengineering Institute, China).
Tissue PKA activity assay
PKA activity was measured according to the method described previously using a nonradioactive PKA assay kit (Promega, USA)14,18. Tissue samples from NA, AR, and ANR were homogenized on ice in PKA extraction buffer containing 25 mmol/L Tris-HCl (pH 7.4), 0.5 mmol/L EDTA, 0.5 mmol/L EGTA, 10 mmol/L β-mercaptoethanol, 1 μg/mL leupeptin, and 1 μg/mL aprotinin. The homogenate was centrifuged at 20 000×g for 5 min at 4 °C, and the supernatant was assayed for PKA activity according to the manufacturer's instructions. The reaction products were separated on a 0.8% agarose gel at 100 V for 15 min. The phosphorylated species migrated toward the positive electrode, whereas the non-phosphorylated substrates migrated toward the negative electrode. The fluorescence intensity of the phosphorylated peptides, which reflects the PKA activity, was quantified by spectrophotometry at 570 nm. One unit of kinase activity is defined as the number of nanomoles of phosphate transferred to a substrate per minute per milliliter.
Western blotting analysis
The expression of Ser133 phosphorylated (pcAMP) response element-binding protein (CREB), eNOS, Ser1179 p-eNOS and Ser635 p-eNOS was detected by Western blotting, as described previously19. Rabbit polyclonal p-CREB (Ser133; 1:100 dilution, Santa Cruz, USA), rabbit polyclonal eNOS (1:250 dilution, Cell Signaling, USA), rabbit monoclonal p-eNOS (Ser1179; 1:250 dilution, Invitrogen, USA), rabbit polyclonal p-eNOS (Ser635; 1:200 dilution, Upstate, USA), or mouse monoclonal β-actin (1:10 000 dilution, Proteintech, USA) antibodies were applied. The immunoreactive bands were visualized using a chemiluminescence reagent. The intensity ratio of the target band to the β-actin band corresponded to the relative amounts of the target protein.
Analysis of tissue NOS activity
Tissue samples from NA, AR, and ANR were homogenized and centrifuged at 3000 r/min for 10 min. The activity of total (t-)NOS, iNOS, and cNOS (the predominantly expressed isoform of cNOS in myocardium is eNOS17) in the supernatant was measured spectrophotometrically at 530 nm according to the manufacturer's instructions (Nanjing KeyGen, China). The activities were expressed as units per milligram of myocardial protein (IU/mg prot).
Statistical analysis
All data are expressed as the mean±SEM. Data from all stages were compared by repeated measures analysis of variance followed by post-hoc analysis with the Student-Newman-Keuls multiple comparisons test. Differences in a single variable data, such as the no-reflow and infarct areas, and the activities of PKA and NOS were compared among groups by ANOVA followed by the Duncan's post hoc test. P<0.05 was considered statistically significant.
Results
Cardiac performance in SIM-treated and -untreated hearts
Physiological examination revealed that there were no significant differences in cardiac hemodynamics between any of the groups at baseline (P>0.05). However, under the conditions of ischemia and reperfusion, HR and LVEDP were increased in the untreated, control hearts (P<0.05). The effects of ischemia and reperfusion were partially diminished when the animals received SIM pretreatment, as HR and LVEDP were decreased in the SIM group (P<0.05). The effects of SIM appeared to depend on the activation of PKA and eNOS because combined treatment with SIM and the PKA inhibitor H-89 or the eNOS inhibitor L-NNA did not have the same effect as treatment with SIM alone (Table 1).
Table 1. Hemodynamic data at baseline, at the end of ischemia, and after reperfusion. Mean±SEM. n=7−8 animals/group. bP<0.05 vs Sham. eP<0.05 vs Control. hP<0.05 vs SIM at the same time point.
| HR (beats/min) | MAP (mmHg) | RPP (mmHg·beats/min) | LVEDP (mmHg) | dp/dtmax (mmHg/s) | dp/dtmin (mmHg/s) | |
|---|---|---|---|---|---|---|
| Baseline | ||||||
| Sham group | 95.4±4.4 | 87.8±7.5 | 8411.8±900.6 | 3.2±0.7 | 1689.8±220.1 | −1309.7±225.5 |
| Control group | 102.6±10.3 | 84.6±10.2 | 8308.7±1023.1 | 4.6±1.1 | 1966.2±168 | −1574.1±121.8 |
| SIM group | 101.9±8.4 | 93.5±7.6 | 9135.5±458.9 | 4.9±1.1 | 1857.1±183.4 | −1581.8±242.0 |
| SIM+H-89 group | 112.7±6.4 | 89.6±4.5 | 9973.1±475.9 | 3.9±0.6 | 2083.4±186.9 | −1434.1±123.9 |
| H-89 group | 112.9±6.9 | 76.1±7.4 | 8621.4±1028.7 | 5.1±1.1 | 1844.9±160.1 | −1309.4±192.7 |
| SIM+L-NNA group | 95.4±8 | 99.1±8.5 | 9371.7±1081.9 | 6±0.3 | 1569.3±192.5 | −1512.2±205.4 |
| L-NNA group | 93.5±5.5 | 102.2±6.9 | 9438.9±630.6 | 5.4±0.5 | 1928.8±157.2 | −1832.2±165.6 |
| Ischemia 90 min | ||||||
| Sham group | 89.4±7.7 | 74.7±5.2 | 6619.0±726.2 | 3.6±0.7 | 1820.7±198.0 | −503.1±397.1 |
| Control group | 126.0±6.2b | 66.1±7.9 | 8544.7±1354.0 | 11.3±3.5b | 1549.4±137.7 | −876.7±108.2 |
| SIM group | 85.6±13.8e | 65.0±2.9 | 6808.0±968.2 | 6.7±1.5 | 1737.8±129.0 | −1096.7±96.9 |
| SIM+H-89 group | 90.3±11.6 | 61.5±3.2 | 4220.0±301.5h | 9.8±2.8 | 1164.4±99.8h | −713.9±107.1 |
| H-89 group | 89.8±7.3e | 70.8±6.1 | 6585.0±1003.5 | 7.5±1.3 | 1389.5±91.2 | −805.2±87.0 |
| SIM+L-NNA group | 100.3±3.2 | 97.1±5.4h | 9754.1±666.8h | 8.3±1.3 | 1262.6±171.7h | −880.4±193 |
| L-NNA group | 103.6±4.4 | 98.4±7.3e | 9208.9±777.8 | 10±1.7 | 1733.1±89.5 | −844.9±74.1 |
| Reperfusion 180 min | ||||||
| Sham group | 69.4±4.9 | 75.4±4.3 | 5215.7±462 | 4.0±1.0 | 1650.3±231.7 | −846.7±105.5 |
| Control group | 80.2±7.8 | 67.0±9.0 | 5354.3±966.4 | 12.8±2.6b | 1236.2±139.6 | −691.9±70.5 |
| SIM group | 80.3±7.1 | 76.0±4.6 | 6131.6±774.2 | 6.8±1.4e | 1718.7±250.0 | −949.1±112.7 |
| SIM+H-89 group | 95.8±11.9 | 59.0±5.8h | 5661.8±859.8 | 7.7±1.6 | 1397.7±183.6 | −786.7±70.1 |
| H-89 group | 84.3±3.4 | 70.4±3.8 | 5963.3±442.9 | 6.9±1.3e | 1385.2±245.3 | −790.3±192.6 |
| SIM+L-NNA group | 79.4±5.3 | 79.3±6.4 | 6338.2±660.8 | 8.8±0.9 | 1013.6±111.9h | −684.2±57.2 |
| L-NNA group | 85.8±5.7 | 82.1±5.3 | 6334±834.5 | 6.2±1.2e | 1265.9±146.6 | −886.7±205.1 |
Abbreviations: SIM=simvastatin; L-NNA=Nω-nitro-L-arginine; HR=heart rate; MAP=mean arterial pressure; RPP=rate-pressure product; LVEDP=left ventricular end-diastolic pressure; dp/dtmax and dp/dtmin=maximum and minimum rates of left ventricular pressure development, respectively.
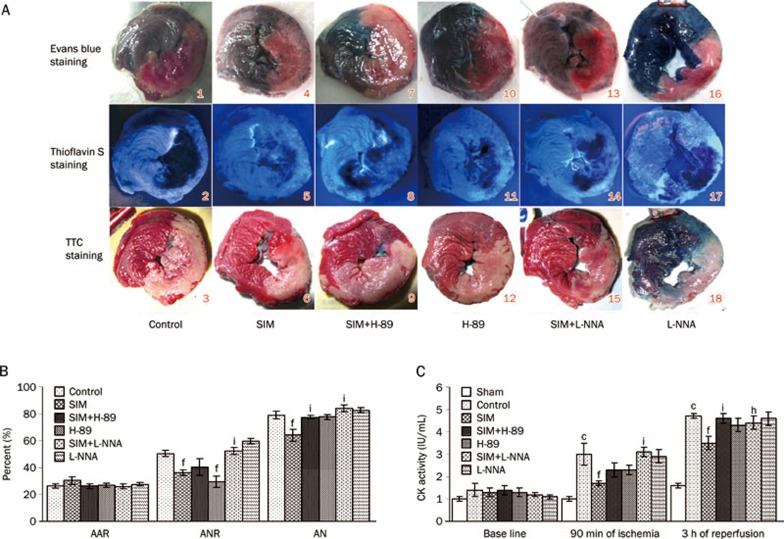
Sizes of no-reflow and infarction after ischemia and reperfusion
Pathological studies revealed that the area at risk (AAR) per left ventricle (LV) was comparable in the control, SIM, SIM+H-89, H-89, SIM+L-NNA, and L-NNA groups, averaging between 26.1% and 30.4% (P>0.05) (Figure 1A and 1B). SIM pretreatment significantly attenuated the area of no-reflow (ANR/AAR, 36.1%±2.1%) and the area of necrosis (AN/AAR, 64.1%±4.5%) compared to the control group (50.4%±2.4%; 79.0%±2.7%) (P<0.01). The PKA inhibitor H-89 alone reduced the no-reflow size (29.5%±4.2%) relative to the control group (P<0.01), but it partially abolished the SIM effect on no-reflow size and completely abolished the SIM effect on infarct size, indicated by the increased no-reflow (40.4%±6.1%) and infarct (77.4%±1.2%) sizes, respectively, in the SIM+H-89 group. However, the eNOS inhibitor L-NNA completely counteracted the effects of SIM on myocardial no-reflow and infarction; the no-reflow and infarction sizes in the SIM+L-NNA group reverted to the control levels (52.3%±2.8%; 83.9%±2.5%) (P<0.01). These data indicate that the cardioprotective effects of SIM against no-reflow and infarction are completely eNOS-dependent but partially PKA-dependent.
Figure 1.
Sizes of area at risk (AAR), area of no-reflow (ANR), and area of necrosis (AN). (A) A representative series of images of the ischemia, no-reflow, and infarction areas at the level of the left ventricle papillary muscle. In the upper panels, the myocardium unstained by Evans blue dye represents AAR. In the middle slices, thioflavin S fluorescent dye negatively stained myocardium indicates the no-reflow area. In the bottom slices, the triphenyltetrazolium chloride (TTC)-unstained white myocardium was identified as the infarct zone. Simvastatin (SIM) pretreatment (4 to 6) decreased the areas of no-reflow and necrosis compared with the control (1 to 3), SIM+H-89 (7 to 9), and SIM+L-NNA (13 to 15) groups. (B) AAR expressed as a percentage of left ventricular mass; ANR and AN expressed as percentages of AAR. SIM significantly reduced the sizes of ANR and AN compared with the control, but these effects disappeared when SIM was coadministered with H-89 or L-NNA. (C) Plasma CK activity assays revealed that SIM eliminated the ischemia-reperfusion-induced elevation of the plasma CK activity after 1.5 h of ischemia and 3 h of reperfusion, while H-89 and L-NNA diminished the effect of SIM treatment. Mean±SEM. n=7–8 animals/group. cP<0.01 vs Sham. fP<0.01 vs Control. hP<0.05, iP<0.01 vs SIM at the same time point.
After 1.5 h of ischemia and 3 h of reperfusion, plasma CK activity, a standard enzymatic marker of cardiac injury, was significantly increased in the control group (2.97±0.45 IU/mL; 4.73±0.14 IU/mL) compared to the sham group (1.05±0.09 IU/mL; 1.59±0.25 IU/mL) (P<0.01) but was lowered in the SIM group (1.66±0.13 IU/mL; 3.53±0.29 IU/mL) compared to the control group (P<0.01). However, the addition of H-89 inhibited the SIM effect after 3 h of reperfusion, and L-NNA inhibited the SIM effect after 1.5 h of ischemia and 3 h of reperfusion (Figure 1C).
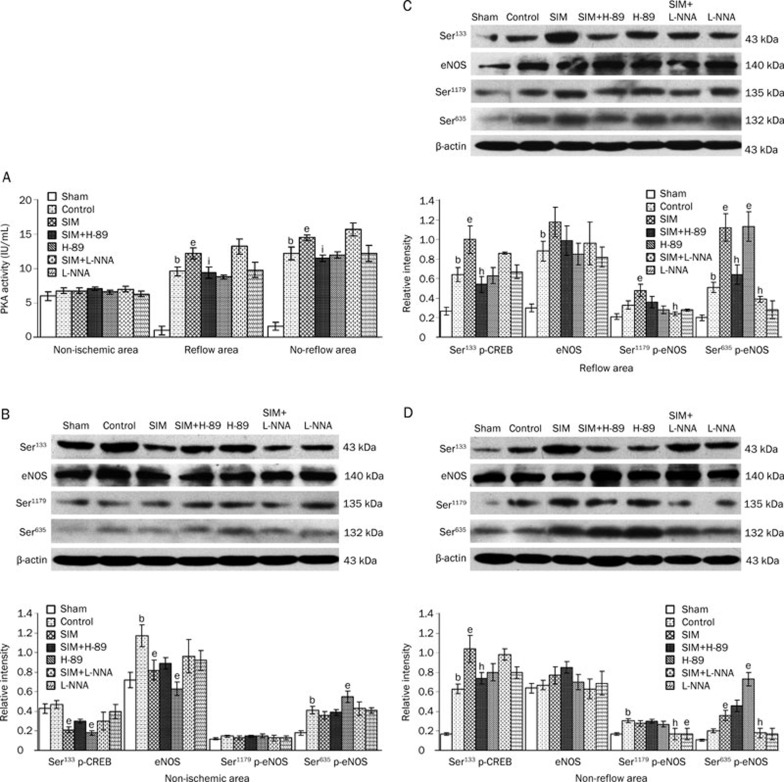
Myocardial PKA activity in the reflow and no-reflow areas after ischemia and reperfusion
Figure 2A shows that the PKA activity was dramatically induced in the reflow and no-reflow areas in the control group (9.57±0.56 IU/mL; 12.18±0.88 IU/mL) compared with that in the sham group (6.04±0.62 IU/mL) (P<0.01). Myocardial PKA activity in the reflow and no-reflow areas were further activated in the SIM group (12.24±0.76 IU/mL; 14.47±0.44 IU/mL) compared to that in the control group (P<0.05). However, SIM-induced PKA activity was inhibited by H-89 but not by L-NNA.
Figure 2.
Myocardial PKA activity and the expression of Ser133 p-CREB, eNOS and p-eNOS (Ser1179 and Ser635) after ischemia and reperfusion. (A) Myocardial PKA activities in the reflow and no-reflow areas were stimulated by ischemia and reperfusion and were further increased by SIM pretreatment. However, the SIM-induced activation of PKA was silenced by H-89 but not by L-NNA. (B) In the non-ischemic area, the expression of eNOS and Ser635 p-eNOS was increased in the control group, and the expression of Ser133 p-CREB and eNOS was decreased in the SIM and H-89 groups. (C) In the reflow area, the expression of Ser133 p-CREB, eNOS, and Ser635 p-eNOS was stimulated by ischemia and reperfusion. SIM promoted the phosphorylation of Ser133 p-CREB, Ser1179 p-eNOS, and Ser635 p-eNOS. However, H-89 counteracted the effects of SIM on Ser133 p-CREB and Ser635 p-eNOS, and L-NNA canceled the effects of SIM on Ser1179 p-eNOS and Ser635 p-eNOS. (D) In the no-reflow area, ischemia and reperfusion induced the phosphorylation of Ser133 p-CREB and Ser1179 p-eNOS. SIM pretreatment increased the phosphorylation of Ser133 p-CREB and Ser635 p-eNOS. H-89 blocked the effect of SIM on Ser133 p-CREB, and L-NNA inhibited the SIM effects on Ser1179 p-eNOS and Ser635 p-eNOS. Mean±SEM. n=7–8 animals/group. bP<0.05 vs Sham. eP<0.05 vs Control. hP<0.05, iP<0.01 vs SIM at the same time point.
To evaluate the inhibition effect of H-89 on the PKA signaling pathway, Western blotting analysis was performed to detect the expression of Ser133 p-CREB, which acts downstream of PKA and is thus an indicator of PKA activity13 (Figure 2B, 2C, and 2D). In the non-ischemic area (Figure 2B), the expression of Ser133 p-CREB was reduced in the SIM and H-89 groups compared to the control group (P<0.05). In the reflow and no-reflow myocardium (Figure 2C and 2D), Ser133 p-CREB was up-regulated in the control group compared to the sham group (P<0.05) and was further activated in the SIM group compared to the control group (P<0.05). The induction effect of SIM on Ser133 p-CREB expression was abolished by H-89 but not by L-NNA (P<0.05). These data suggest that the PKA pathway was activated during ischemia and reperfusion and was further stimulated by SIM pretreatment.
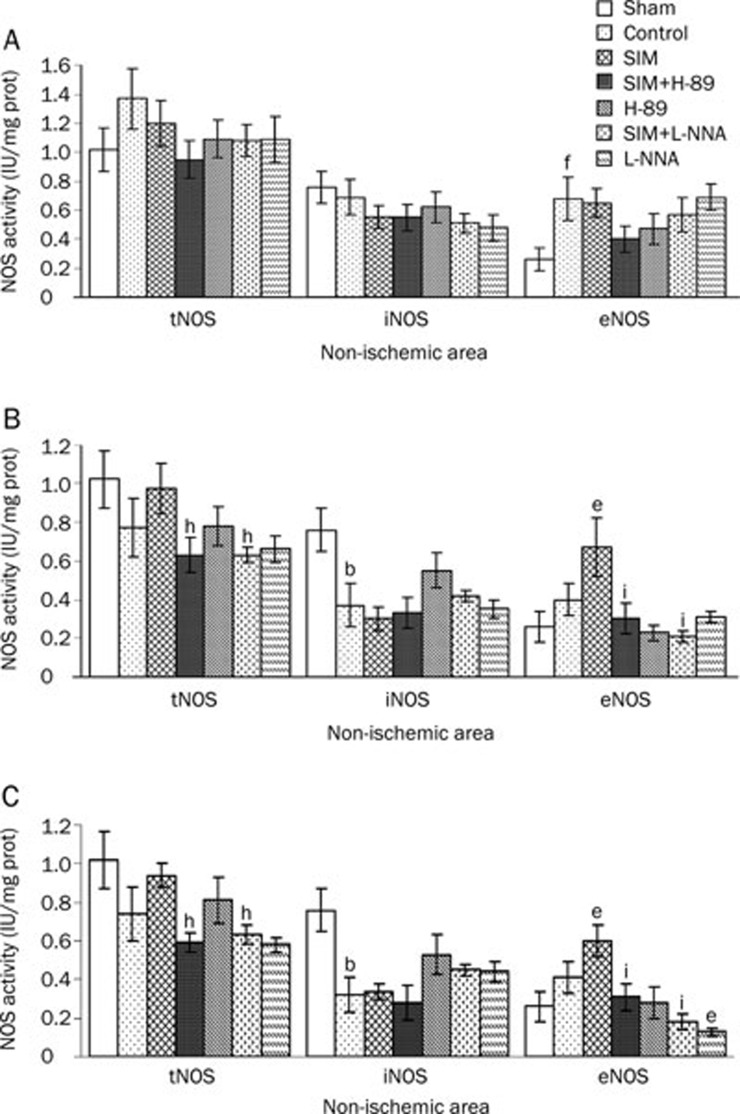
Myocardial NOS activities in the reflow and no-reflow areas after ischemia and reperfusion
In the non-ischemic area (Figure 3A), cNOS activity was increased in the control group (0.68±0.15 IU/mg prot) relative to the sham group (0.26±0.08 IU/mg prot) (P<0.01). In the reflow and no-reflow areas (Figure 3B and 3C), tNOS activity was decreased in the SIM+H-89 (0.63±0.09 IU/mg prot; 0.59±0.05 IU/mg prot) and SIM+L-NNA (0.73±0.06 IU/mg prot; 0.73±0.05 IU/mg prot) groups relative to the SIM group (0.97±0.13 IU/mg prot; 0.94±0.06 IU/mg prot) (P<0.05); iNOS activity was decreased in the control group (0.37±0.08 IU/mg prot; 0.32±0.09 IU/mg prot) relative to the sham group (0.76±0.11 IU/mg prot) (P<0.01); and cNOS activity was increased in the SIM group (0.67±0.15 IU/mg prot; 0.6±0.08 IU/mg prot) relative to the control group (0.4±0.11 IU/mg prot; 0.41±0.08 IU/mg prot) (P<0.05) but decreased in the SIM+H-89 (0.3±0.08 IU/mg prot; 0.31±0.07 IU/mg prot) and SIM+L-NNA (0.28±0.07 IU/mg prot; 0.2±0.04 IU/mg prot) groups compared to the SIM group (P<0.01).
Figure 3.
Myocardial NOS activity in the injured and uninjured myocardium after ischemia and reperfusion. (A) After ischemia and reperfusion, myocardial constitutive (cNOS) activities were increased in the non-ischemic area in the control group. (B and C) In the reflow and no-reflow myocardium, inducible (iNOS) activity was suppressed in the control group. SIM pretreatment significantly stimulated cNOS activity, but this effect was abolished by cotreatment with H-89 or L-NNA. Abbreviation: tNOS=total nitric oxide synthase. Mean±SEM. n=7–8 animals/group. bP<0.05 vs Sham. eP<0.05, fP<0.01 vs Control. hP<0.05, iP<0.01 vs SIM at the same time point.
To investigate the mechanism by which PKA mediates eNOS activity, the expression of eNOS and p-eNOS (Ser1179 and Ser635) was detected by Western blotting analysis (Figure 2B, 2C, and 2D). In the non-ischemic area (Figure 2B), the expression of eNOS and Ser635 p-eNOS was increased in the control group compared to that in the sham group (P<0.05); the eNOS expression in the SIM and SIM+H-89 groups was decreased, and the Ser635 p-eNOS expression in the H-89 group was increased, compared to that of the control group (P<0.05). In the reflow area (Figure 2C), the expression of eNOS and Ser635 p-eNOS was increased in the control group compared with that in the sham group (P<0.05); the Ser1179 p-eNOS and Ser635 p-eNOS phosphorylation was increased in the SIM group compared to that in the control group (P<0.05); H-89 suppressed the induction effect of SIM on Ser635 p-eNOS (P<0.05), and L-NNA suppressed the induction effect of SIM on both Ser1179 p-eNOS and Ser635 p-eNOS (P<0.05). In the no-reflow area (Figure 2D), Ser1179 p-eNOS phosphorylation was higher in the control group than in the sham group (P<0.05). The level of Ser635 p-eNOS was increased in the SIM and H-89 groups compared to that of the control group (P<0.05), whereas the levels of Ser1179 p-eNOS and Ser635 p-eNOS were decreased in the SIM+L-NNA group (P<0.05).
Discussion
The main findings of our study include the following: first, a single-dose SIM pretreatment just 2.5 hours before reperfusion reduced the sizes of the no-reflow and necrosis areas and activated the PKA pathway and the phosphorylation of eNOS at Ser635 and Ser1179 in the reflow and no-reflow myocardium. Second, the PKA inhibitor H-89 blocked the SIM-induced PKA activation and partially abolished the SIM-induced cardioprotection and eNOS phosphorylation, whereas the eNOS inhibitor L-NNA completely blocked the SIM-induced cardioprotection and eNOS phosphorylation without any influence on PKA activity, indicating that the cardioprotection of SIM after ischemia and reperfusion is in part mediated by the PKA/eNOS pathway.
Previous studies have reported that a 3-day pretreatment with atorvastatin or SIM at 10 mg/kg per day decreased the infarct size in rat hearts, but this effect was not observed at 2 mg/kg4,6,20,21. Similarly, acute pretreatment with high-dose SIM (10 μmol/L) was shown to attenuate the ischemia-reperfusion injury in isolated rat hearts, but chronic treatment of low-dose SIM did not22. These data suggest that chronic or acute pretreatment with high-dose statins can attenuate infarct size after ischemic reperfusion. The effect of SIM on infarct size in this study is consistent with that found in previous studies because 2 mg/kg of SIM in pigs is approximately equivalent to 10 mg/kg in rats, after correction for body surface area15. The infarct-limitation effect of statins in ischemia-reperfusion is mainly attributed to its pleiotropic effects via the PI3K/Akt/eNOS pathway because the effects of the statins can be abolished by inhibiting PI3K or eNOS5,6,7,8,9. In this study, we further found that acute pretreatment with single-dose SIM not only decreased the infarct size but also attenuated the no-reflow area, and we showed that the PKA pathway was another important mediator in the cardioprotection of SIM that acts by modulating the phosphorylation of eNOS at Ser1179 and Ser635.
PKA seems to be activated by endogenous mechanisms because the PKA inhibitor H89 alone had almost the same effect as SIM on the no-reflow area in the presence of ischemia. The mechanism underlying the idiopathetic activation of PKA is considered to be that the decreased level of cyclic guanosine monophosphate (cGMP) inhibits the activity of phosphodiesterase III upon reperfusion, which in turn increases the cAMP concentration, subsequently leading to PKA activation23. The inhibition of PKA activity and Ser133 p-CREB by H-89 indicates that acute SIM treatment actually results in PKA activation. Although it is presently unclear how statins activate PKA in ischemic myocardium, one probable explanation is that statins may stimulate a cell surface receptor that activates small G proteins, which results in the sensitization of adenylate cyclase and the accumulation of myocardial cAMP24 and eventually the activation of PKA in the ischemic myocardium. It has been reported that 5′-nucleotidase and the adenosine A1, A2A, and A2B receptors are involved in atorvastatin-induced eNOS phosphorylation by stimulating phospholipase A2 and cyclooxygenase (COX) to generate prostacyclin-2 (PGI2), leading to PKA activation and subsequent eNOS phosphorylation5,25.
Enhancing Ser1177/1179 phosphorylation via the PI3K/Akt pathway is considered to be the main mechanism by which statins protect against ischemia-reperfusion injury5,6,7,8,9. However, several lines of evidence have shown that PKA also regulates the phosphorylation of eNOS at Ser1179, Ser635, and Ser617 in bovine eNOS (Ser1177, Ser633/635, and Ser615 in humans)24,26,27,28,29. Ser633 phosphorylation is critical in maintaining NO synthesis after the initial sensitization by Ca2+ flux and Ser1177 phosphorylation30, and it is stimulated via the PKA pathway in response to shear stress and acute statin treatment in aortic endothelial cells24,27, suggesting that PKA-mediated Ser633/635 phosphorylation may be another mechanism by which statins protect against myocardial no-reflow and necrosis after ischemia and reperfusion. Our study confirmed this hypothesis in reperfused swine hearts, demonstrating that the inhibition of PKA partially blocked the SIM-induced phosphorylation of eNOS at Ser1179 and Ser635 in the reflow and no-reflow myocardium, as well as partially abrogated the effects of SIM against myocardial no-reflow and infarction. Previous studies have reported that statin-induced eNOS activation involves the inhibition of the Rho GTPase and the modulation of Rho A membrane translocation31,32 and that the transient preischemic activation of PKA by ischemic preconditioning reduces infarct size through Rho and Rho-kinase (ROCK) inhibition during sustained ischemia13. Therefore, it is plausible that PKA-Rho pathway is involved in the regulation of eNOS activity by statins, but the specific mechanism should be studied further.
Here, H-89 administered 30 min before ischemia attenuated the no-reflow area, possibly by phosphorylating eNOS at Ser635, but partly inhibited the protective effects of SIM when infused 30 min after SIM administration. This finding is somewhat contradictory to previous studies. In isolated rat hearts, H-89 (2 μmol/L) improved postischemic function and decreased infarct size when injected 3 min before 30 min of global ischemia-reperfusion and further reduced the infarct size when administered 3 min prior to ischemic or forskolin (a cAMP-elevating agents) preconditioning33. However, when delivered at 1.35 μg/kg per minute in dogs or at 10 μmol/L in isolated rat hearts approximately 30 min before preconditioning, H-89 completely blunted the infarct-limitation effect of preconditioning12,34. Therefore, the partial inhibition of H-89 on SIM cardioprotection in our study most likely occurred because H-89 was delivered later than SIM, and the contradiction between these studies might be explained by the differences in H-89 dosage, experimental protocols, and animal species.
Another mechanism underlying the bidirectional role of H-89 in ischemia and reperfusion may be the cross-talk between the PKA and PI3K/Akt pathways in the regulation of eNOS phosphorylation. It has been reported that H-89 inhibits Akt, ROCK II, and 5′-AMP-activated protein kinase (AMPK)35 and that the PKA pathway interacts with the PI3K/Akt pathway in the regulation of gene expression36. Previous studies have shown that the forskolin-induced stimulation of PKA can inhibit Akt activity in human embryonic kidney cells37, but epinephrine or forskolin-induced stimulation of PKA enhanced eNOS phosphorylation at Ser1177 by activating the Akt pathway in aortic or coronary endothelial cells28,38. Interestingly, in endothelial cells, PKA is mainly involved in eNOS phosphorylation during the early phase of preconditioning, whereas both PKA and Akt are required for late preconditioning-induced eNOS activation, and Akt is a substrate of PKA39. Therefore, these reports indicate that PKA plays different roles in regulating eNOS phosphorylation in different cells and that cross-talk most likely exists between the PKA and PI3K/Akt pathways in the regulation of eNOS phosphorylation during ischemia and reperfusion. The inhibition of PKA may in turn cause the activation of the PI3K/Akt pathway and the subsequent phosphorylation of Ser635 p-eNOS. This might be another explanation of why H-89 partially inhibited SIM-induced cardioprotection in our study; the exact mechanism may be elucidated in the future when more selective PKA inhibitors are available.
In summary, the present study suggests that acute pretreatment with a single dose of SIM just 2.5 h before reperfusion can attenuate the size of the no-reflow and infarction areas by phosphorylating eNOS at Ser1179 and Ser635 in a partially PKA-dependent manner. The observation that H-89 partially abolished the cardioprotective effects of SIM and decreased the no-reflow size when administered alone suggests a bidirectional role for PKA in cardioprotection during ischemia and reperfusion. Our results are helpful for understanding the mechanisms involved in statin-mediated protection against myocardial no-reflow and infarction, and may lead to the development of new criteria for treating patients undergoing acute post-infarct percutaneous coronary intervention.
Author contribution
Yue-jin YANG, Xiang-dong LI, and Yi-ling WU designed research; Xiang-dong LI, Jing-lin ZHAO, Hai-tao ZHANG, and Yu-tong CHENG performed research and analyzed data; Xiang-dong LI and Yong-jian GENG wrote the paper.
Acknowledgments
This study was supported by the National Basic Research Program (973 Program) of China (No 2012CB518602 and 2011CB503901).
References
- Ito H, Maruyama A, Iwakura K, Takiuchi S, Masuyama T, Hori M, et al. Clinical implications of the 'no reflow' phenomenon. A predictor of complications and left ventricular remodeling in reperfused anterior wall myocardial infarction. Circulation. 1996;93:223–8. doi: 10.1161/01.cir.93.2.223. [DOI] [PubMed] [Google Scholar]
- Stone GW, Peterson MA, Lansky AJ, Dangas G, Mehran R, Leon MB. Impact of normalized myocardial perfusion after successful angioplasty in acute myocardial infarction. J Am Coll Cardiol. 2002;39:591–7. doi: 10.1016/s0735-1097(01)01779-x. [DOI] [PubMed] [Google Scholar]
- Resnic FS, Wainstein M, Lee MK, Behrendt D, Wainstein RV, Ohno-Machado L, et al. No-reflow is an independent predictor of death and myocardial infarction after percutaneous coronary intervention. Am Heart J. 2003;145:42–46. doi: 10.1067/mhj.2003.36. [DOI] [PubMed] [Google Scholar]
- Zhao JL, Yang YJ, Cui CJ, You SJ, Gao RL. Pretreatment with simvastatin reduces myocardial no-reflow by opening mitochondrial K(ATP) channel. Br J Pharmacol. 2006;149:243–9. doi: 10.1038/sj.bjp.0706862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merla R, Ye Y, Lin Y, Manickavasagam S, Huang MH, Perez-Polo RJ, et al. The central role of adenosine in statin-induced ERK1/2, Akt, and eNOS phosphorylation. Am J Physiol Heart Circ Physiol. 2007;293:H1918–28. doi: 10.1152/ajpheart.00416.2007. [DOI] [PubMed] [Google Scholar]
- Manickavasagam S, Ye Y, Lin Y, Perez-Polo RJ, Huang MH, Lui CY, et al. The cardioprotective effect of a statin and cilostazol combination: relationship to Akt and endothelial nitric oxide synthase activation. Cardiovasc Drugs Ther. 2007;21:321–30. doi: 10.1007/s10557-007-6036-0. [DOI] [PubMed] [Google Scholar]
- Iwakura K, Ito H, Kawano S, Okamura A, Kurotobi T, Date M, et al. Chronic pre-treatment of statins is associated with the reduction of the no-reflow phenomenon in the patients with reperfused acute myocardial infarction. Eur Heart J. 2006;27:534–9. doi: 10.1093/eurheartj/ehi715. [DOI] [PubMed] [Google Scholar]
- Efthymiou CA, Mocanu MM, Yellon DM. Atorvastatin and myocardial reperfusion injury: new pleiotropic effect implicating multiple prosurvival signaling. J Cardiovasc Pharmacol. 2005;45:247–52. doi: 10.1097/01.fjc.0000154376.82445.06. [DOI] [PubMed] [Google Scholar]
- Birnbaum Y, Ye Y, Rosanio S, Tavackoli S, Hu ZY, Schwarz ER, et al. Prostaglandins mediate the cardioprotective effects of atorvastatin against ischemia-reperfusion injury. Cardiovasc Res. 2005;65:345–55. doi: 10.1016/j.cardiores.2004.10.018. [DOI] [PubMed] [Google Scholar]
- Heusch G, Kleinbongard P, Bose D, Levkau B, Haude M, Schulz R, et al. Coronary microembolization: from bedside to bench and back to bedside. Circulation. 2009;120:1822–36. doi: 10.1161/CIRCULATIONAHA.109.888784. [DOI] [PubMed] [Google Scholar]
- Van de Werf F, Bax J, Betriu A, Blomstrom-Lundqvist C, Crea F, Falk V, et al. Management of acute myocardial infarction in patients presenting with persistent ST-segment elevation: the Task Force on the Management of ST-Segment Elevation Acute Myocardial Infarction of the European Society of Cardiology. Eur Heart J. 2008;29:2909–45. doi: 10.1093/eurheartj/ehn416. [DOI] [PubMed] [Google Scholar]
- Inserte J, Garcia-Dorado D, Ruiz-Meana M, Agullo L, Pina P, Soler-Soler J. Ischemic preconditioning attenuates calpain-mediated degradation of structural proteins through a protein kinase A-dependent mechanism. Cardiovasc Res. 2004;64:105–14. doi: 10.1016/j.cardiores.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Sanada S, Asanuma H, Tsukamoto O, Minamino T, Node K, Takashima S, et al. Protein kinase A as another mediator of ischemic preconditioning independent of protein kinase C. Circulation. 2004;110:51–7. doi: 10.1161/01.CIR.0000133390.12306.C7. [DOI] [PubMed] [Google Scholar]
- Li XD, Yang YJ, Geng YJ, Jin C, Hu FH, Zhao JL, et al. Tongxinluo reduces myocardial no-reflow and ischemia-reperfusion injury by stimulating the phosphorylation of eNOS via the PKA pathway. Am J Physiol Heart Circ Physiol. 2010;299:H1255–61. doi: 10.1152/ajpheart.00459.2010. [DOI] [PubMed] [Google Scholar]
- Freireich EJ, Gehan EA, Rall DP, Schmidt LH, Skipper HE. Quantitative comparison of toxicity of anticancer agents in mouse, rat, hamster, dog, monkey, and man. Cancer Chemother Rep. 1966;50:219–44. [PubMed] [Google Scholar]
- Cheng YT, Yang YJ, Zhang HT, Qian HY, Zhao JL, Meng XM, et al. Pretreatment with Tongxinluo protects porcine myocardium from ischaemia/reperfusion injury through a nitric oxide related mechanism. Chin Med J(Engl) 2009;122:1529–38. [PubMed] [Google Scholar]
- Brahmajothi MV, Campbell DL. Heterogeneous basal expression of nitric oxide synthase and superoxide dismutase isoforms in mammalian heart: implications for mechanisms governing indirect and direct nitric oxide-related effects. Circ Res. 1999;85:575–87. doi: 10.1161/01.res.85.7.575. [DOI] [PubMed] [Google Scholar]
- Wu ZQ, Li M, Chen J, Chi ZQ, Liu JG. Involvement of cAMP/cAMP-dependent protein kinase signaling pathway in regulation of Na+,K+-ATPase upon activation of opioid receptors by morphine. Mol Pharmacol. 2006;69:866–76. doi: 10.1124/mol.105.016501. [DOI] [PubMed] [Google Scholar]
- Robinet A, Hoizey G, Millart H. PI3-kinase, protein kinase C, and protein kinase A are involved in the trigger phase of beta1-adrenergic preconditioning. Cardiovasc Res. 2005;66:530–42. doi: 10.1016/j.cardiores.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Ye Y, Lin Y, Perez-Polo R, Huang MH, Hughes MG, McAdoo DJ, et al. Enhanced cardioprotection against ischemia-reperfusion injury with a dipyridamole and low-dose atorvastatin combination. Am J Physiol Heart Circ Physiol. 2007;293:H813–8. doi: 10.1152/ajpheart.00210.2007. [DOI] [PubMed] [Google Scholar]
- Birnbaum Y, Lin Y, Ye Y, Merla R, Perez-Polo JR, Uretsky BF. Pretreatment with high-dose statin, but not low-dose statin, ezetimibe, or the combination of low-dose statin and ezetimibe, limits infarct size in the rat. J Cardiovasc Pharmacol Ther. 2008;13:72–9. doi: 10.1177/1074248407312839. [DOI] [PubMed] [Google Scholar]
- Szarszoi O, Maly J, Ostadal P, Netuka I, Besik J, Kolar F, et al. Effect of acute and chronic simvastatin treatment on post-ischemic contractile dysfunction in isolated rat heart. Physiol Res. 2008;57:793–6. doi: 10.33549/physiolres.931559. [DOI] [PubMed] [Google Scholar]
- Schulz R, Kelm M, Heusch G. Nitric oxide in myocardial ischemia/reperfusion injury. Cardiovasc Res. 2004;61:402–13. doi: 10.1016/j.cardiores.2003.09.019. [DOI] [PubMed] [Google Scholar]
- Harris MB, Blackstone MA, Sood SG, Li C, Goolsby JM, Venema VJ, et al. Acute activation and phosphorylation of endothelial nitric oxide synthase by HMG-CoA reductase inhibitors. Am J Physiol Heart Circ Physiol. 2004;287:H560–6. doi: 10.1152/ajpheart.00214.2004. [DOI] [PubMed] [Google Scholar]
- Ray CJ, Marshall JM. The cellular mechanisms by which adenosine evokes release of nitric oxide from rat aortic endothelium. J Physiol. 2006;570:85–96. doi: 10.1113/jphysiol.2005.099390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boo YC, Sorescu G, Boyd N, Shiojima I, Walsh K, Du J, et al. Shear stress stimulates phosphorylation of endothelial nitric-oxide synthase at Ser1179 by Akt-independent mechanisms: role of protein kinase A. J Biol Chem. 2002;277:3388–96. doi: 10.1074/jbc.M108789200. [DOI] [PubMed] [Google Scholar]
- Boo YC, Hwang J, Sykes M, Michell BJ, Kemp BE, Lum H, Jo H. Shear stress stimulates phosphorylation of eNOS at Ser(635) by a protein kinase A-dependent mechanism. Am J Physiol Heart Circ Physiol. 2002;283:H1819–28. doi: 10.1152/ajpheart.00214.2002. [DOI] [PubMed] [Google Scholar]
- Zhang XP, Hintze TH. cAMP signal transduction induces eNOS activation by promoting PKB phosphorylation. Am J Physiol Heart Circ Physiol. 2006;290:H2376–84. doi: 10.1152/ajpheart.00614.2005. [DOI] [PubMed] [Google Scholar]
- Michell BJ, Harris MB, Chen ZP, Ju H, Venema VJ, Blackstone MA, et al. Identification of regulatory sites of phosphorylation of the bovine endothelial nitric-oxide synthase at serine 617 and serine 635. J Biol Chem. 2002;277:42344–51. doi: 10.1074/jbc.M205144200. [DOI] [PubMed] [Google Scholar]
- Mount PF, Kemp BE, Power DA. Regulation of endothelial and myocardial NO synthesis by multi-site eNOS phosphorylation. J Mol Cell Cardiol. 2007;42:271–9. doi: 10.1016/j.yjmcc.2006.05.023. [DOI] [PubMed] [Google Scholar]
- Rikitake Y, Liao JK. Rho GTPases, statins, and nitric oxide. Circ Res. 2005;97:1232–5. doi: 10.1161/01.RES.0000196564.18314.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulhak A, Roy J, Hedin U, Sjoquist PO, Pernow J. Cardioprotective effect of rosuvastatin in vivo is dependent on inhibition of geranylgeranyl pyrophosphate and altered RhoA membrane translocation. Am J Physiol Heart Circ Physiol. 2007;292:H3158–63. doi: 10.1152/ajpheart.01354.2006. [DOI] [PubMed] [Google Scholar]
- Makaula S, Lochner A, Genade S, Sack MN, Awan MM, Opie LH. H-89, a non-specific inhibitor of protein kinase A, promotes post-ischemic cardiac contractile recovery and reduces infarct size. J Cardiovasc Pharmacol. 2005;45:341–7. doi: 10.1097/01.fjc.0000156825.80951.14. [DOI] [PubMed] [Google Scholar]
- Sanada S, Kitakaze M, Papst PJ, Asanuma H, Node K, Takashima S, et al. Cardioprotective effect afforded by transient exposure to phosphodiesterase III inhibitors: the role of protein kinase A and p38 mitogen-activated protein kinase. Circulation. 2001;104:705–10. doi: 10.1161/hc3201.092216. [DOI] [PubMed] [Google Scholar]
- Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soria LR, Gradilone SA, Larocca MC, Marinelli RA. Glucagon induces the gene expression of aquaporin-8 but not that of aquaporin-9 water channels in the rat hepatocyte. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1274–81. doi: 10.1152/ajpregu.90783.2008. [DOI] [PubMed] [Google Scholar]
- Mei FC, Qiao J, Tsygankova OM, Meinkoth JL, Quilliam LA, Cheng X. Differential signaling of cyclic AMP: opposing effects of exchange protein directly activated by cyclic AMP and cAMP-dependent protein kinase on protein kinase B activation. J Biol Chem. 2002;277:11497–504. doi: 10.1074/jbc.M110856200. [DOI] [PubMed] [Google Scholar]
- Kou R, Michel T. Epinephrine regulation of the endothelial nitric-oxide synthase: roles of RAC1 and beta3-adrenergic receptors in endothelial NO signaling. J Biol Chem. 2007;282:32719–29. doi: 10.1074/jbc.M706815200. [DOI] [PubMed] [Google Scholar]
- Bellis A, Castaldo D, Trimarco V, Monti MG, Chivasso P, Sadoshima J, et al. Cross-talk between PKA and Akt protects endothelial cells from apoptosis in the late ischemic preconditioning. Arterioscler Thromb Vasc Biol. 2009;29:1207–12. doi: 10.1161/ATVBAHA.109.184135. [DOI] [PubMed] [Google Scholar]