Abstract
There is burgeoning interest in the study of positive emotion regulation and psychopathology. Given the significant public health costs and the tremendous variance in national prevalence rates associated with many disorders of positive emotion, it is critical to reach an understanding of how cultural factors, along with biological factors, mutually influence positive emotion regulation. Progress in this domain has been relatively unexplored, however, underscoring the need for an integrative review and empirical roadmap for investigating the cultural neuroscientific contributions to positive emotion disturbance for both affective and clinical science domains. The present paper thus provides a multidisciplinary, cultural neuroscience approach to better understand positive emotion regulation and psychopathology. We conclude with a future roadmap for researchers aimed at harnessing positive emotion and alleviating the burden of mental illness cross-culturally.
Keywords: Emotion Regulation, Positive Emotion, Psychopathology, Culture, Neuroscience
Introduction
There is burgeoning interest in the study of emotion regulation and psychopathology (e.g., Kring & Sloan, 2009). Growing interest has mounted more recently to better understand both the regulation of positive emotion (e.g., Dillon & Pizzagalli, 2009; Gruber, Mauss, & Tamir, 2011) and its neural underpinnings (Ochsner & Gross, 2008). Making progress in this domain is particularly important for studying emotional disorders, whose phenomenology and etiology primarily feature disturbances in positive emotion. Given the significant public health costs and the tremendous variance in national prevalence rates associated with many emotional disorders, it is critical to reach an understanding of how cultural factors, along with more well-studied biological factors, mutually influence positive emotion regulation. Unfortunately, progress in this domain has been left relatively unexplored, underscoring the need for a theoretical framework and empirical roadmap for studying universality and cultural specificity of positive emotional processes in both affective and clinical science domains.
In the present paper, we aim to provide this critically needed multidisciplinary approach into the nature of positive emotion regulation and psychopathology through the lens of a cultural neuroscience approach (Chiao & Ambady, 2007; Chiao et al., 2010; Chiao, 2011). We do so by first providing a foundational background into the nature of positive emotion regulation by illustrating how this process is disrupted in key disorders characterized by core deficits in positive emotion. Second, we provide insight into understanding the etiology and public health importance of psychopathology as characterized by population mental health disparities in these key disorders. Third, we provide an overview of a cultural neuroscience approach and discuss its translation to the domains of both positive emotion regulation and psychopathology. We conclude in our fourth section by integrating a cultural neuroscience approach with the study of positive emotion regulation and related psychopathologies.
Positive Emotion Regulation (PER) and Psychopathology
Emotion regulation refers to the processes by which individuals consciously or unconsciously modify their emotions (Gross & Thompson, 2007). The ability to adaptively regulate emotion has been linked to favorable health outcomes, including greater well-being and social adjustment (Tamir, John, Srivastava, & Gross, 2007; Tugade & Fredrickson, 2004). Although the benefits of PER are clear, only recently have researchers discussed potential difficulties in how individuals harness positive emotions (e.g., Gruber et al., 2011). A promising approach to better understand difficulties in PER is to examine individuals who exhibit disturbances in positive emotion (e.g., Gruber & Keltner, 2007). In this paper, we review the etiology of four candidate emotional disorders that are characterized by disturbance in PER and may also be subject to some degree of cultural influence: bipolar disorder (BD) and alcohol use disorders (AUDs), which are characterized in part by relative amplifications in positive emotion, as well as major depressive disorder (MDD) and social anxiety disorder (SAD), which are characterized in part by relative diminutions in positive emotion.1
BD
Bipolar Disorder is a severe and chronic psychiatric disorder associated with serious functional and social impairment (Dilsaver, 2011) and has been ranked as one of the top ten causes of worldwide disability. The core diagnostic criterion for BD involves disrupted affective functioning, critically including periods of abnormally and persistently elevated positive mood (i.e., hypomania/mania) (American Psychiatric Association, 2000). Empirical work further highlights the central role of PER disturbance in uniquely differentiating BD—characterized by heightened reactivity and difficulty down-regulating positive emotions—from other disorders (e.g., Gruber, 2011; Gruber, Harvey, & Purcell, 2011; Johnson, Gruber, & Eisner, 2007).
With respect to emotion reactivity, BD is associated with a persistent pattern of heightened positive emotional reactivity that is independent of context (e.g., Gruber, 2011) and is independent of current symptom levels (Johnson, 2005). For example, individuals with BD self-report greater positive emotion (PE) in response to neutral photos (M’Bailara et al., 2009) and at the prospect of earning rewards in their daily lives (Meyer, Johnson, & Winters, 2001) compared to healthy controls. Individuals at high risk for, and diagnosed with, BD also self-reported greater PE to both positive and negative emotional stimuli (Gruber, Johnson, Oveis, & Keltner, 2008; Gruber, Harvey, & Purcell, 2011; Meyer & Baur, 2009). Experience-sampling studies further suggest that individuals at high risk for BD report greater PE across situations in daily life (Hofmann & Meyer, 2006). Research investigating physiological responses indicates that high risk BD individuals demonstrate elevated levels of cardiac vagal tone— a putative parasympathetic marker of PE and resilience—compared to low risk participants (Gruber et al., 2008). Finally, neuroimaging studies reveal that BD patients, compared to healthy controls, exhibit increased activity in the orbitofrontal cortex (Elliott et al., 2004), a region associated with PE, in response to positive photos of human smiles. With respect to emotion regulation, studies suggest that BD involves difficulties in down-regulating PE (Green, Cahill, & Malhi, 2007; Gruber, 2011; Gruber et al., 2008; Johnson et al., 2007). Specifically, people with BD exhibit prolonged PE after a positive mood induction relative to controls (Farmer et al., 2006). Not only do individuals with BD have difficulties down-regulating positive emotional responses, but they also have a tendency to up-regulate or amplify PE, referred to as positive rumination (Gruber, Eidelman, Johnson, Smith, & Harvey, in press).
Importantly, emerging evidence suggests that BD patients who exhibit more pronounced disturbance in PER experience worse clinical health outcomes. For example, several studies suggest that self-reported sensitivity to positive stimuli predicts increases in manic symptoms in BD over time (Johnson, 2005; Meyer et al., 2001). Greater reports of positive feelings like joy predict increased manic symptom severity in BD (Gruber et al., 2009). These findings suggest that increased levels of PE are themselves (independent of disturbances in negative emotion and distress) associated with a worse illness course in BD. This suggests that PER in BD is an important candidate for further inquiry to better understand the important role of culture in mental health.
AUDs
As the world’s third largest risk factor for disease burden, alcohol consumption is a salient global health issue (World Health Organization (WHO), 2011). Alcohol use disorders (AUDs), including both alcohol abuse and dependence, involve expending considerable time and energy towards obtaining and consuming alcohol, despite recurring problems due to its consumption (American Psychiatric Association, 2000). Importantly, AUDs have been linked to difficulties in PE regulation (Regier et al., 1990; Swendsen et al., 1998; Schneider et al., 2001). Specifically, they have been tied to increased PE reactivity during alcohol consumption, decreased PE reactivity to non-alcohol stimuli, and the inability to successfully regulate heightened craving of alcohol. This may lead addicts towards continued consumption of alcohol as an emotion-altering strategy.
With respect to reactivity, one line of work reveals increased PE signaling (i.e., liking or enjoyment) during alcohol consumption, including self-reported euphoria and relaxation (Morgan & Badawy, 2001). Neuroimaging evidence further reveals that alcohol use stimulates activation of the reward-related neural circuitry associated with reward and pleasure, including the striatum, nucleus accumbens, and caudate (Gilman, Ramchandani, Davis, Bjork, & Hommer, 2008; Schreckenberger et al., 2004). Alcohol use also increases dopaminergic cell firing, a process commonly associated with pleasurable experiences (Gessa, Muntoni, Collu, Vargui, & Mereu, 1985). Given that PE indicates a benefit to the organism, increased PE during alcohol consumption in AUDs may provide a false signal of benefit and lead to increased alcohol use despite negative consequences (Nesse & Berridge, 1997). A second line of work on emotion reactivity and AUDs suggests that it is associated with decreased reactivity to non-alcohol cues. Positively valenced photos with no alcohol content are rated as less arousing by subjects with AUDs, indicating a blunted reactivity to these pleasurable pictures (Aguilar de Arcos, Verdejo-Garcia, Peralta-Ramirez, Sanchez-Barrera, & Perez-Garcia, 2005). Neuroimaging data further suggests that those with AUDs exhibit decreased ventral striatum activity, which is commonly associated with PE, to non-alcohol cues such as monetary rewards (Beck et al., 2009; Wrase et al., 2007), and that addicts exhibit reduced sensitivity of dopaminergic receptors to non-alcohol cues (Dettling et al., 1995). Such reduced sensitivity to non-alcohol stimuli may foster maintenance of long-term addiction, as it may drive individuals with AUDs to seek out alcohol in order to compensate for a lack of PE from other sources (Koob & Le Moal, 1997).
With respect to PER, AUDs are associated with the inability to regulate intense desires or cravings centered on alcohol-relevant cues (Tiffany, 1990). The wanting of alcohol seems central to addiction, as individuals with AUDs who were recently administered alcohol continue to report craving—their experienced PE in response to alcohol consumption may actually exacerbate, rather than satisfy, their craving (Meyer, 1988). In Robinson and Berridge’s (1993, 2003) incentive-sensitization theory of drug and alcohol addiction, those with AUDs are driven to consume alcohol due to alterations in the neural system that render it hypersensitive to the “wanting” of these drinking experiences, independent of the “liking” of them, and those who find themselves seeking alcohol despite negative consequences are unable to regulate this hypersensitized craving. Furthermore, recurrent alcohol drinking may serve as a behavioral regulator to amplify or enhance PE (Wills & Shiffman, 1985).
Clinically, dysregulated PER may underlie the onset of AUDs (Cheetham, Allen, Yucel, & Lubman, 2010). Specifically, as alcohol-related cues may instigate a dysregulated craving response and lead to relapse (Robinson & Berridge, 2008), we may improve clinical outcomes of this globally-prevalent disorder by understanding why those with AUDs have trouble down-regulating PE in response to alcohol-related cues, and trouble up-regulating PE to other non-alcohol related stimuli.
MDD
MDD is a common, chronic, and recurrent emotional disorder marked by disruptions in PER (e.g., Rottenberg, Gross, & Gotlib, 2005) and considered the most prevalent of mental health disorders across both high and middleto-low income countries (Collins et al., 2011). A core symptom of MDD includes difficulty generating and/or maintaining PE (e.g., anhedonia; American Psychiatric Association, 2000). Current models of MDD highlight core deficits in diminished PE that uniquely differentiate MDD from other forms of psychopathology, such as anxiety disorders (e.g., Kring & Sloan, 2009; Rottenberg, Gross, & Gotlib, 2005; Watson, Clark, & Carey, 1998).
With respect to emotion reactivity, those with MDD experience diminished PE reactivity at both trait and state levels. Participants with MDD also report lower PE in their daily life period compared to healthy controls (Lovejoy & Steuerwald, 1995; Peeters, Nicholson, & Berkhof, 2003). Meta-analyses conducted across 19 laboratory studies comparing emotion reactivity of individuals with MDD to healthy controls suggest that MDD is generally associated with reduced PE reactivity (Bylsma, Morris, & Rottenberg, 2008). For example, in response to amusing films, MDD participants exhibited fewer positive facial expressions and reported lower PE compared to controls (Rottenberg, Kasch, Gross, & Gotlib, 2002; Berenbaum & Oltmanns, 1992). In response to positive photos (e.g., people smiling, nature scenes, erotica), MDD participants reported feeling less PE, less emotionally aroused, and displayed less intense facial expressions (Allen, Trinder, & Brennen, 1999; Dunn, Dalgleish, Lawrence, Cusack, & Ogilvie, 2004; Sloan, Strauss, & Wisner, 2001). Furthermore, MDD is associated with decreased left as compared to right frontal lobe activation, biological correlates corroborating diminished positive emotional reactivity (e.g., Davidson, Abercrombie, Nitschke, & Putnam, 1999; Davidson, Pizzagalli, Nitschke, & Putnam, 2002).
With respect to emotion regulation, individuals with MDD exhibit difficulty up-regulating and sustaining positive feelings (Forbes & Dahl, 2005; Garber, Braafladt, & Weiss, 1995; McMakin, Santiago, & Shirk, 2009). For example, individuals with MDD self-report difficulty increasing PE (Garnefski & Kraaij, 2006; Sloan et al., 2001). Individuals with a history of MDD are also unable to effectively use a positive autobiographical memory recall to improve or enhance their current mood state (Joormann, Siemer, & Gotlib, 2007). Individuals with mild depression symptoms exhibit a decreased duration of PE following the offset of positive film stimuli (McMakin et al., 2009). Finally, neuroimaging data suggests that those with MDD failed to sustain activity in the nucleus accumbens associated with PE when attempting to increase positive feelings (Heller et al., 2009).
Clinically, the ability to exhibit PER in MDD is related to mental health outcomes. For example, diminished behavioral and psychophysiological responding to positive stimuli predicted a lower probability of recovery from depressive symptoms (Rottenberg et al., 2002). Moreover, decreased self-reported behavioral approach tendencies—associated with the pursuit of reward-related motivational behavior—predict greater concurrent depression severity and worse prospective prognosis (Rottenberg et al., 2002). Thus, the loss of appropriate and flexible PE responding in MDD reflects a core feature of emotion dysregulation (Rottenberg, Gross, & Gotlib, 2005; Rottenberg et al., 2002).
SAD
Although reduced PE has classically been a factor that differentiates depression from anxiety (Watson, Clark, & Carey, 1988; Clark, Watson, & Mineka, 1994), emerging evidence points to an important role of reduced PE in anxiety disorders as well. We focus here on social anxiety disorder (SAD) because its association with reduced PE has been highly discussed (e.g., Brown, Chorpita, & Barlow, 1998; Kashdan, 2007), and because culture plays an important role in how emotion is experienced and expressed in the disorder (Okazaki, Liu, Longworth, & Minn, 2002; Lee, Okazaki, & Yoo, 2006)—indeed, prevalence of SAD varies widely cross-culturally (Kessler & Ustun, 2008). We discuss growing evidence that associates SAD with reduced PE reactivity and an inability to seek and cultivate PE from potentially pleasurable situations.
With respect to emotion reactivity, findings point to an association between social anxiety symptoms and diminished PE reactivity (Brown et al., 1998). Notably, a meta-analysis across 19 studies found that greater social anxiety predicted decreased PE, independent of current depression severity (Kashdan, 2007). SAD has also been associated with lower outward expression of PE compared to healthy controls (Turk, Heimberg, Luterek, Mennin, & Fresco, 2005). In everyday life, trait social anxiety predicts decreased time spent feeling happy and decreased peak intensity of PE (Kashdan & Collins, 2010). Finally, individuals with SAD may not reap the same benefits from socializing because they interpret social events in a less positive light (Kashdan, Weeks, & Savostyanova, 2011) and experience decreased PE (Kashdan & Roberts, 2007).
With respect to emotion regulation, few studies to date have directly examined PER in SAD. However, recent findings highlight the importance of this topic. First, symptoms of SAD themselves can lead individuals to choose situations that decrease or dampen PE. For example, fear of others’ evaluations may lead individuals with SAD to avoid otherwise pleasurable social situations (Herbert, Rheingold, & Brandsma, 2010; Kashdan & Steger, 2006). The association between low PE and SAD bears important clinical implications including functioning and quality of life (Kashdan & Collins, 2010). With evidence that PE is linked to essential social components such as interpersonal trust (Burns et al., 2008), and relationships (Fredrickson, 1998), low PE may be one of the barriers preventing individuals with SAD from achieving positive interpersonal outcomes. In sum, SAD is associated with diminished reactivity to positive stimuli and difficulty generating PE, especially in social situations.
Transdiagnostic Considerations
Before proceeding, it is important to acknowledge that, although the above section assigns specific PER disturbances to particular disorders, this division is primarily a means of organization, rather than a strict rule. Indeed, disruptions in PER may actually generalize across many different diagnoses, consistent with a body of literature on transdiagnostic processes in clinical psychology (e.g., Harvey, Watkins, Mansell, & Shafran, 2004; Kring, 2008). Researchers who take this transdiagnostic perspective on disorder argue that a set of similar emotional, cognitive, biological, and social mechanisms are common to a broad range of disorders rather than specific to any particular one. Indeed, within the emotional domain most generally, disruptions in core affect, emotional awareness, and emotion regulation have been shown `to characterize a broad range of disorders, ranging from personality to sleep to mood disorders (Kring, 2008). With respect to the particular role of PE in disorder, other researchers (e.g., Dillon & Pizzagalli, 2009) similarly begin by presenting disorder-specific evidence on PE dysfunction, but use this framework as a heuristic, acknowledging the transdiagnostic aspects of these processes. For example, although they characterize MDD as involving a disruption in some of the normal functions of PE, Dillon and Pizzagalli (2009) emphasize that other disorders, such as those typically characterized more primarily by deficits in regulating PE (e.g., SUDs), also involve some elements of anhedonia (Leventhal et al., 2008). Moreover, with respect to BD, Merikangas and colleagues (2007) have proposed that BD exists along a spectrum, with traits along this spectrum existing both in those with and without psychiatric diagnoses. This raises the question of precisely when positive emotion becomes maladaptive along the continuum of PE experience, and whether this boundary differs between cultures. In such a way, different components of PER can be said to be broadly relevant to mental health, rather than solely characteristic of one disorder. We find that our disorder-specific approach serves as simply a useful heuristic to begin building a cultural neuroscience conception of PER, though we do not deny the future need to expand this focus.
Importantly, the transdiagnostic perspective on disorder predicts that the biological underpinnings of psychopathology—in addition to the emotional processes on which we currently focus—are similarly cross-cutting.2 With respect to biological mechanisms that we have presently reviewed, there is indeed some evidence for such a claim. For example, just as symptoms of different reward-related processes (anhedonia on one hand, and rewardrelated sensitivity on the other) are shared across several disorders, so too—to some degree—are the neural correlates of these symptoms. As reviewed, patients with MDD have shown deficits in sustaining NAcc activity when instructed to increase PE. Similarly, anhedonic symptoms in schizophrenia are associated with decreased activity in NAcc during reward anticipation. Furthermore, alterations in the DA reward system—different in kind from those in depression, but similar in the general circuitry implicated—are also seen in patients with BPD and SUDs. Despite these commonalities, however, it is important to note that in all these cases, slight differences in the neuroimaging task (e.g., anticipation of monetary reward, experience of social reward, or emotion regulation) and the particular reward regions implicated (VS versus OFC, for example) may still suggest consideration of relevant differences between patient groups. Indeed, we argue that ultimately there exist differences in prevalence of mental health disorders across cultures and that distinct kinds of psychological and neural mechanisms give rise to such distinct disorders (Kessler & Ustun, 2008). For instance, the prevalence of clinical anxiety is typically associated with phenotypes such as increased vigilance for fearful events or objects in the environment and brain regions, such as the human amygdala, are specialized for perceiving and recognizing objects and events in the environment that may lead to anxiety (Ohman & Mineka, 2001).
Cultural Influences on Psychopathology and PER
In the first section, we demonstrated that PE regulation plays a critical role in understanding the etiology of mental health disorders. Much of the empirical work on the universality and cultural specificity of PE regulation and related psychopathology has been conducted primarily in Western populations (Henrich, Heine, Norenzayan, 2008; Chiao & Blizinsky, 2010). Nevertheless, a growing body of research indicates that culture affects how people perceive, experience and regulate PE (Leu, Wang, & Koo, 2011; Miyamoto & Ma, 2011; Tsai, Knutson, & Fung, 2006).
In this second section we build a strong rationale for understanding how and why including culture is critical to understanding disorders of PE. We first describe the global prevalence rates of our four key disorders (i.e., BD, AUDs, MDD, and SAD) in order to highlight the potential for influence of culture on PER in these disorders. Next, we demonstrate how culture affects three key features of PER broadly, including how a person would like to feel, the regulation strategies utilized to manage emotion, and the degree to which one up-regulates PE. This section will thus provide a background into understanding the role of culture in both psychopathology and PER, providing fertile territory towards articulating the cultural neuroscience model of PER and psychopathology that will follow later in our discussion.
Global prevalence of key emotional disorders
Prevalence rates of psychopathology are high across the globe. For example, in a worldwide survey conducted by the WHO, 14.2% of interviewed participants reported symptoms of one or more DSM-IV disorders in the past year alone (Alonso et al., 2011). However, the lifetime prevalence of psychopathology ranges from 12% in Nigeria and 13% in China, to 39% in New Zealand and 46% in the United States (Kessler & Ustun, 2008). As applied to the present paper, we highlight the prevalence of the four key disorders and associated population mental health disparities. This discussion highlights the need for considering culture when examining disorders of PER. In addition, each of these disorders demonstrates distinct prevalence rates across different cultures, pointing to potential sources of cross-cultural variation in the experience and expression of PE. We briefly highlight the prevalence rates of these four key disorders below as an entry point into understanding the contribution of cultural influences to the experience and expression of psychopathology.
BD
The percentage of respondents worldwide who report symptoms of BD in the past year is 1.6% (Alonso et al., 2011). Importantly, there is considerable variation between prevalence rates for BD in Western compared to East Asian countries. Specifically, the lifetime prevalence of BD has been found to be three times higher in the U.S. compared to Taiwan, and twice as high in the U.S. compared to Korea (Weissman et al., 1996).
AUDs
The worldwide 12-month prevalence rate for AUDs is 1.8% (Alonso et al., 2011); lowest in Italy (0.2%) and Japan (1%), and highest in Ukraine (5.5%). Ukraine seems to be at particular risk for AUDs, such that 13.5% of interviewees report alcohol abuse in their lifetime (Kessler & Ustun, 2008). Previous studies have consistently reported low rates of alcohol abuse in East Asian countries including China, Taiwan, and Japan (Chen et al., 1993; Kawakami, Shimizu, Haratani, Iwata, & Kitamura, 2004). Interestingly, of all the disorders measured in the World Mental Health surveys, AUDs are the only one in which country poverty level seems to increase risk (Alonso et al., 2011).
MDD
MDD has a worldwide 12-month prevalence rate of 5.8% (Alonso et al., 2011). The highest lifetime prevalence of MDD was found in the Netherlands (17.9%) and the lowest rates in African and East Asian nations, including 3.1% in Nigeria (Kessler & Ustun, 2008) 2.9% in Korea, and 1.5% in Taiwan (Weissman et al., 1996). Low rates of MDD in East Asian nations have been consistently noted (Chen et al., 1993; Lee et al., 1990). MDD prevalence varies not only country to country, but also within the U.S., where the lifetime prevalence of MDD was found to be 6.9% in Chinese Americans (Takeuchi et al., 1998), which is 60% lower than the U.S. national average. The finding that the Chinese American prevalence rate falls part way in between that of Chinese living in China (the lowest) and that of the national U.S. rate (the highest) suggests that acculturation into the U.S. corresponds with increasing MDD prevalence rates—further evidence of culture’s influence on psychopathology.
SAD
For SAD, the worldwide 12-month prevalence is 2.7% (Alonso et al., 2011). Rates of SAD are considerably lower in East Asian countries such as Japan (0.5%) and China (0.3%) as compared to the U.S. (6.8%; Kessler & Ustun, 2008). As in our other three key disorders, we see considerably lower prevalence rates in East Asians than in European Americans. SAD prevalence also varies within the U.S., as Caucasians have a much higher rate of SAD (12.6%) than Asian Americans (5.3%;) (Asnaani, Richey, Dimaite, Hinton, & Hofmann, 2010). Similar to MDD, Asian American prevalence rates fall part way in between that of Asians living in Asia (the lowest) and that of European Americans (the highest), again suggesting that acculturation into the U.S. corresponds with increasing SAD prevalence rates.
Taken together, these findings highlight the severity and prevalence of four key disorders on a global scale and the importance of understanding the etiology of population mental health disparities. One particularly salient theme is that of the consistently low mental illness prevalence rates in East Asian compared to Western industrialized nations. Convergent evidence from anthropology and cultural neuroscience indicate that cultural values, practices and beliefs may serve as a protective factor against developing PER-related disorders (Chiao & Blizinsky, 2010; Dressler, Balieiro, Ribeiro, & Dos Santos, 2009). What cultural factors might be particularly important or unique to Asian culture and how it influences the experience and expression of PER? We now turn to discussing cultural influences on PE as a basis for beginning to form an integrative cultural neuroscience model that illuminates the intersection of psychopathology, PER and culture.
Cultural influences on PE
Understanding cultural influences on PE and PER is a necessary ingredient in order to understand how PER goes awry in emotional disorders that exhibit variable prevalence rates cross-culturally. We thus highlight three key factors or types of cultural influences on PE. First, culture influences the types of emotional goals people set, or how people want to feel (i.e., “ideal affect”; Tsai et al., 2006) which is particularly apparent in the case of PE (Miyamoto & Ma, 2011). For example, when asked to report how often they would ideally want to experience a variety of positive feelings, Westerners reported wanting to experience high arousal positive (HAP) states, such as enthusiasm or elation, more often relative to Easterners, who in contrast more often wanted to experience low arousal positive (LAP) states, such as feeling peaceful or calm (e.g., Tsai et al., 2006). Importantly, these ideal affect preferences influence the specific kinds of affective experiences that people seek out in order to actively produce and amplify these kinds of specific PE goals (Miyamoto & Ma, 2011; Tsai, 2007).
Second, culture influences the types of regulation processes that are selected to modulate experiences of PE. For example, members of cultures that emphasize social hierarchy and interpersonal embeddedness (e.g., Eastern cultures) are more likely to report greater use of emotion regulation strategies such as behavioral suppression— defined as an outward masking of facial or bodily emotional displays—when regulating both positive and negative emotions (Matsumoto, Yoo, Nakagawa, et al., 2008). Members of cultures that are less permissive of strong displays of emotion tend also to report feeling a less extreme range of emotion (e.g., Mesquita & Karasawa, 2002). By contrast, members of cultures that are more permissive of strong displays of emotion (e.g., Western cultures) are more likely to endorse cognitive reappraisal or rethinking affective experiences in order to achieve optimal affective states (Matsumoto, Yoo, Fontaine, et al., 2008).
Third, the actual tendency to regulate one’s PE differs cross-culturally, including the tendency to up-regulate or maintain positive feelings (also referred to as savoring). In a series of studies, Miyamoto & Ma (2011) found that European Americans tend to savor PE whereas East Asians tend to dampen, or down-regulate, PE. Specifically, when recalling an important success, European Americans are more likely to report a greater number of savoring strategies and outcomes (e.g., attending a fun party), had less intention to dampen PE, and experienced a higher ratio of positive relative to negative emotions on the day after their initial academic success. East Asians, on the other hand, were more likely to report having used dampening strategies, such as thinking about events that may decrease PE level. This East Asian preference for dampening PE may originate from a dialectical theory of emotion in which positive and negative emotions are believed to be mixed as opposed to opposite—thus making PEs less unambiguously positive, and less preferably savored.
To summarize thus far, we have described cross-national differences in the prevalence of emotional disorders, which further underscores the importance of considering culture. We then demonstrated that cultural values shape a number of aspects of PE experience, including ideals surrounding which PEs one wants to feel and the ways that individuals engage in PER. How can we integrate these findings to understand PER and psychopathology in general, and on a broader level globally, in a way that is sensitive to cultural variation? We now turn to the third section below in which we introduce an integrative cultural neuroscience perspective on understanding PER and its relationship to psychopathology.
Cultural Neuroscience Perspective on PER
Thus far, we have demonstrated the important role that PER plays in four key types of psychopathology (BD, AUDs, MDD, and SAD). We have also demonstrated that culture influences global prevalence rates in these disorders, and that culture influences PER more basically. Importantly, this triangular relationship suggests that we cannot fully understand these disorders of PER without understanding the mediating role of culture—that is, because have shown that culture influences both mental illnesses (seen in the cross-national variations in the expression of psychopathology) as well as PE (seen in the variations in PE processing across cultures). In order to more fully understand PE and its related disorders, there is a need to examine them through a cultural lens.
We posit that an optimal method with which to study the interaction between culture, PE, and psychopathology is cultural neuroscience. To this end, we first introduce and define cultural neuroscience and its goals, mention its benefits over traditional methods of studying emotion and psychopathology, and outline the specific cultural neuroscience tools that are most relevant to developing a cultural approach towards the four disorders of PE regulation discussed earlier in the paper. In our discussion of each of our cultural neuroscience methods, we connect the clinical findings from these key disorders to possible directions for future cultural neuroscience investigations.
What is cultural neuroscience?
The cultural neuroscience framework seeks to examine how cultural values, practices, and beliefs modulate neural and genetic processes related to human psychology and behavior. Research in cultural neuroscience integrates genetic, neural and cultural levels of analysis to address how and why behavioral outcomes, such as population health disparities in emotion functioning, arise (Chiao & Ambady, 2007; Chiao, 2011; Chiao, Hariri, et al., 2010; see Figure 1 from Chiao & Immordino-Yang, in press; Chiao & Blizinsky, under review). Given the dynamic nature of culture-biology interactions (Li, 2003), a key goal in cultural neuroscience research is to understand the interplay of cultural and biological factors across multiple situational, lifespan and evolutionary time scales (Chiao, 2011; Chiao & Ambady, 2007; Chiao et al., 2010). Understanding the sources and underlying reasons for observed differences and commonalities between cultural groups is an important rationale for cultural neuroscience research (Ambady & Bharucha, 2009) across the lifespan (Park & Gutchess, 2006).
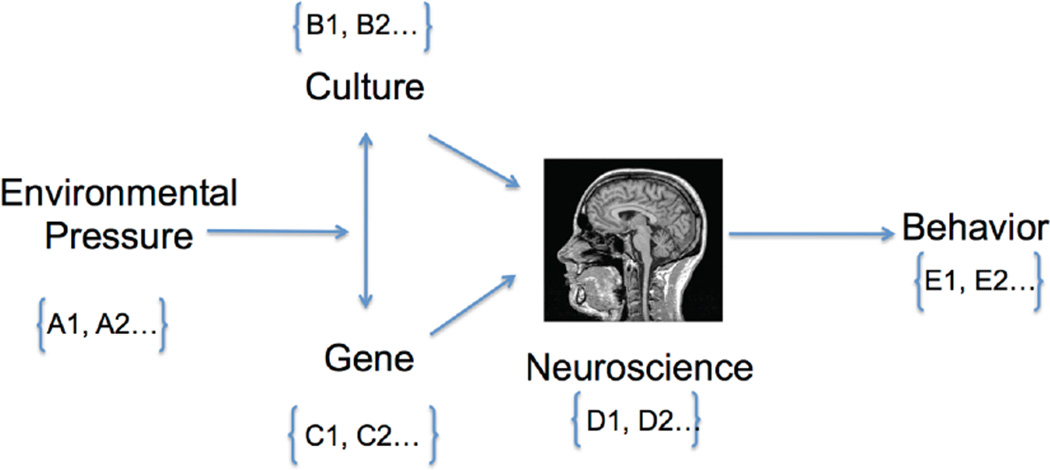
Figure 1.
Cultural neuroscience model of behavior (adapted from 38; Chiao & Blizinsky, under review). Cultural (B1, B2) and genetic adaptations (C1, C2) arise in response to environmental pressures (A1, A2), which then shape neural responding (D1, D2) and ultimately behavior (E1, E2). Here, the behavioral outcomes of interest (E1, E2) are positive emotion regulation (PER) and mental health outcome (i.e., presence or absence of psychopathology).
Why is cultural neuroscience important?
Taking the sociocultural context into account in psychological studies helps to understand the human mind and behavior in a more comprehensive way, beyond the narrow scope of Western, industrialized nations (Arnett, 2002; Henrich, Heine, & Norenzayan, 2010). It allows scientists to parse apart those facets of behavior that are culturally specific from those that are universal. The relationship between culture and the brain is regarded as bidirectional: just as neurobiological functioning adapts to cultural practices, cultural practices adapt to neurobiological constraints (Ambady & Bharucha, 2009; Park & Gutchess, 2006). Due to the potential for culture to significantly influence the human mind and brain, Zhou and Caccioppo (2010) suggest an ongoing integration of the two because of “the inextricable links between sociocultural and biological levels of organization.” Understanding how culture influences brain structure and function may enable us not only to merge the scientific study of how cultural and biological mechanisms give rise to human behavior, but also to provide a broader, more inclusive representation of human nature in non-Western nations, where empirical studies within the behavioral and brain sciences are not yet fully incorporated (Chiao & Cheon, 2010).
An additional benefit of a cultural neuroscience approach to emotion regulation is the opportunity to measure PER and related processes with greater objectivity. Many current cross-cultural comparison studies are conducted solely with behavioral measures, such as open-ended interview and self-report methods (e.g., Kessler & Ustun, 2008). Relying exclusively on these methods to understand affective disorders in any population is prone to the limitations of subjectivity, and even more so when conducting cross-cultural research. Cultural neuroscience allows researchers to avoid such limitations, and thus may be a more optimal method with which to compare PER processes across cultures. Additionally, cross-cultural neuroimaging may be enable investigators to disentangle the blurry distinction between PE reactivity and PER (McRae, Ochsner, Mauss, Gabrieli, & Gross, 2008). Behavioral or survey methods capture the holistic emotional response, but alone, they do not differentiate the reactivity component from the regulation component (Silvers et al., 2012). Because neuroimaging and psychophysiology allow us to indirectly measure brain response (e.g., Dowd & Barch, 2012), these methods may be better suited for understanding the complexities of PE responding across cultures. Notably, neuroscience may provide a further advantage for providing a better understanding of the etiology in population mental health disparities by allowing researchers to better understand the influence of culture at a more proximate level of analysis (Figure 1). Given that human behavior is a byproduct of psychological and neural processes that are dynamically shaped by culture, it is important for us to be able to investigate not only the distal influence of culture on behavior, but also the proximate influence on intermediate or endophenotypic mechanisms of positive emotion and related behaviors.
Methods of cultural neuroscience
Cultural neuroscience integrates methodological tools from neuroscience, genetics, cultural psychology, and anthropology (Chiao, Hariri, et al., 2010). For our purposes, we focus specifically on those cultural neuroscience tools that are likely to be most effective in examining emotion processing and psychopathology, and are thus potentially most relevant for developing a cultural approach towards PE and its related disorders (See Table 1).
Table 1.
Cultural Neuroscience Approach to Studying PER and Psychopathology
| Level of Analysis | Key Methodology | Key Dependent Variables |
|---|---|---|
| Cultural | Self-Report Surveys on Culture |
|
| Neural | fMRI |
|
| Genetic | Behavioral Genetics |
|
| Psychophysiology | Parasympathetic Nervous System Activity |
|
| Behavioral | Self-Report Surveys on Emotion |
|
| FACS |
|
Note: PER = Positive Emotion Regulation which refers to the process of maintaining, upregulating, or downregulating positive emotion; fMRI = Functional Magnetic Resonance Imaging which provides an indirect measure of neural activity; VTA = ventral tegmental area; nACC = nucleus accumbens; OFC = orbitofrontal cortex; VS = ventral striatum; mPFC = medial prefrontal cortex; dlPFC = dorsolateral prefrontal cortex; 5-HTTLPR = Serotonin Transporter Linked Polymorphic Region, long (L) and short (S) alleles; DRD4 = Dopamine Receptor D4, long (L) and short (S) alleles refer to variants of a polymorphic region within the exon III region of the DRD4 gene; RSA = Respiratory Sinus Arrhythmia; PA = Positive Affect; NA = Negative Affect; FACS = Facial Action Coding System.
Measurement of Culture
The primary step in any cultural neuroscience investigation is to accurately measure culture—that is, to assess participants’ cultural values, practices, and beliefs. To do this, culturally-appropriate surveys and behavioral assays are incorporated into study design, with the ultimate goal of understanding how sociocultural contexts affect people’s ability to maintain psychological well-being (Chiao, Hariri, et al., 2010). Quite commonly, cultural psychologists use of measures of cultural values that may predict emotional behavior, such as Self Construal Style (SCS; Singelis, 1994) or way of conceiving of one’s self in relation to others (that is, as either independent and separate from others, or interdependent with others). Due to the importance of ensuring that cultural measurements are accurate, however—that is, that they are interpreted by members of multiple cultures in a similar fashion and accurately capture the essence of an individual’s cultural values, practices and beliefs—cultural neuroscience studies may also include efforts to ensure survey validity, such as back-translation (Brislin, 1970), behavioral methods such as situation-sampling (Kitayama et al., 1997), and mixed methods that incorporate qualitative and quantitative measures of cultural phenomena (Lieber & Weisner, 2010).
Measurement of cultural values is central to investigating the cultural shaping of the brain. In particular, recent work in cultural neuroscience aims to correlate and even determine the causal influence of cultural values on brain function. For example, cultural values of individualism and collectivism, as measured by the SCS scale noted above, indeed shape a broad range of cognitive and emotional neural mechanisms. For instance, individualists and collectivists tend to have different ways of viewing the self, and this distinction appears to be instantiated at the neural level as well; specifically, individualists and collectivists both preferentially recruit medial prefrontal cortex (MPFC)—a region involved in self-related processing—when thinking about themselves in a culturally congruent versus incongruent way (Chiao et al., 2009). In individuals with bicultural Asian-American identities, temporarily heightening awareness of one cultural identity or another through means of priming results in increased neural response within MPFC and other similarly-functioning cortical midline regions, such as the posterior cingulate cortex (PCC), but only for culturally congruent means of self-judgment (Chiao, Harada, et al., 2010). Similarly, cultural values of individualism-collectivism also modulate amygdala response to negative emotional scenes. Compared to Caucasian-Americans, Native Japanese show increased amygdala response when perceiving emotional scenes. Additionally, when bicultural Asian-Americans are primed with collectivism compared to individualism, they similarly show increased amygdala response, indicating that cultural values, rather than ethnicity per se, affect neural mechanisms underlying emotional response (Chiao et al., in revision). Overall, as used in the domain of cultural neuroscience, the measurement of cultural values allows us to isolate the psychological factors which contribute to different patterns of cognition and emotion. Future work is needed to elucidate the effects of culture on the neural basis of PER specifically—our next subsection further explores this possibility.
Neuroimaging
Neuroscience methods, such as functional magnetic resonance neuroimaging (fMRI), provide important ways for measuring neural response with high spatial resolution. In cultural neuroscience, neuroimaging seeks to disentangle those neural substrates that are specific to certain cultural backgrounds from those that are invariant across cultures (Chiao, Hariri, et al., 2010; Han & Northoff, 2008; Park & Gutchess, 2002). Previous studies of cultural influence on emotion processing suggest that there may exist key brain regions of interest that will show cultural variation in neural bases of PER and psychopathology. Prior neuroimaging studies have indeed identified several regions that underlie emotion regulation and reactivity. However, the neural mechanisms of PE regulation have not yet been compared across cultural groups—thus limiting our knowledge to the mere finding that regulation differences exist, while leaving us agnostic as to how they are realized. We focus, therefore, on defining key candidate regions implicated in emotional processing that can be used for future cultural neuroscientific study of PER and its disorders.
Basic PE, such as reward, has its basis in dopamine neurotransmission originating in the ventral tegmental area (VTA), which projects to regions such as the nucleus accumbens (NAcc), amygdala, hippocampus, and medial prefrontal cortex (mPFC) (Berridge & Kringelbach, 2008). The PE of reward is also associated with increased activity in the orbitofrontal cortex (OFC), insula, anterior cingulate cortex, and ventral striatum (Berridge & Kringelbach, 2008; Knutson, Fong, Adams, Varner, & Hommer, 2001; Kringelbach, 2005). Important for process dissociation, the anticipatory phase of reward processing—linked to motivation and goal-directed behavior—and the consummatory phase of reward—linked to subjective feelings of pleasure and satiation—are neutrally distinct. Despite prior evidence that people demonstrate varying degrees of sensitivity to reward cues due to both individual (Kuhnen & Chiao, 2009) and cultural differences (Weber & Hsee, 2000), little is known about how culture affects neural bases of basic positive emotion. This is critical given associations between increased activity in reward-related centers such as the ventral striatum and OFC in individuals with BD as compared to healthy controls (Almeida, Versace, Hassel, Kupfer, & Phillips, 2010; Elliott et al., 2004; Lawrence et al., 2004; Yurgelun-Todd et al., 2000). Examination of cultural influences on the neural basis of emotion may provide important clues as to whether culture may serve to exacerbate PER in BD, for example, by amplifying reward sensitivity in U.S. cultures that value high arousal positive emotions (e.g., Tsai et al, 2006). Likewise, Asian cultures that place a high premium on low arousal positive emotions may serve to promote successful down-regulation of heightened positivity in BD.
With respect to PER, previous neuroimaging studies have found a regulatory relationship between regions of the prefrontal cortex (PFC) and subcortical regions, e.g., the amygdala, that are involved in generating negative emotion (Ochsner & Gross, 2008; Phillips, Ladouceur, & Drevets, 2008). The deployment of regulatory strategies is associated with increased activity in prefrontal regions implicated in cognitive control (Nee, Wager, & Jonides, 2007; Wager, Reading, & Jonides, 2004), simultaneous with decreased activity in emotion-processing subcortical regions. Given the regulatory association between the PFC and subcortical regions associated with negative emotion, it is plausible that a comparable neural relationship exists between the PFC and subcortical regions such as VS and the OFC that are associated with PE. Furthermore, maintaining culturally prescribed levels of ideal affect may be achieved using similar circuitry underlying the up-regulation of emotion (McRae, Misra, Prasad, Pereira, & Gross, 2011). In terms of our key emotional disorders, neurobiological research may help shed light on the way culture differentially mediates the neurobiological reactivity to alcohol consumption in AUDs in comparison to the way it mediates regulation of craving. Learning more about this plausible relationship, and how it differs among cultures, may improve our understanding of PE-related psychopathological traits that vary cross-culturally: for example, the ability to regulate craving of alcohol.
In sum, given the differential importance of adherence to display norms across cultures, it is likely that cultural values play key roles in understanding the biological bases of positive emotion regulation. However, little is known to date about individual or cultural variation in the neural bases of PER. Indeed, there is a dire need to illustrate the shaping of brain function by culture, specifically with regards to PER. This literature does not yet exist, but substantial evidence has been collected regarding the capacity for culture to influence both brain function (e.g., Chiao & Ambady, 2007; Chiao, Hariri, et al., 2010; Chiao, 2011) and emotion regulation strategy, therefore making this aspect of our model not only plausible, but an important start in understanding cultural differences in prevalence of mental illness. We furthermore acknowledge that our concept of mental illness can be shaped by culture, and while it is outside of the scope of this discussion to fully review this point, we now acknowledge this important possibility (Chiao & Cheon, in press).
Genetic polymorphisms
Empirical research in culture-gene coevolution seeks to understand how both cultural and genetic selection affects the human mind, brain and behavior (Boyd & Richeson, 1983; Chiao & Bilizinsky, 2010). A majority of prior neurogenetics work (Brown & Hariri, 2006; Hariri & Weinberger, 2003; Munafò, Brown, & Hariri, 2008) and behavioral genetics research (Caspi Hariri Holmes, Uher, & Moffitt, 2010) seeks to understand how genes affect neural and behavioral correlates of negative emotion processing. Given population genetics evidence that ethnic groups vary in their frequency distribution for specific functional polymorphisms (Novembre & Di Rienzo, 2009; Rosenberg et al, 2010), it is likely that genetic distance across ethnic groups may produce neural and cultural distance across individuals. Recently, behavioral genetics research identifying specific genes that regulate positive emotion have gained momentum. We outline two specific genetic polymorphisms of interest that have previously been implicated in cross-cultural differences in emotion processing that may enable us to better understand PE and its regulation.
The first genetic polymorphism of interest is a polymorphic region within the promoter region of the serotonin transporter gene (5-HTTLPR) —it is found to contribute to affective differences and is also known to vary in allelic frequency across cultures. In Western populations, individuals who carry at least one copy of the “short” (S) compared to the long (L) allele of this gene show increased negative emotion responding (Chiao & Blizinsky, 2010). For example, carriers of the S allele show increased amygdala responding associated with heightened emotion intensity and salience in the presence negative emotional stimuli (Hariri et al., 2002; Munafo et al., 2008) and are more likely to experience negative affect when under stress (Caspi et al., 2003). Importantly, behavioral genetics researchers have shown that East-Asians and collectivistic nations are significantly more likely to carry the S allele of the serotonin transporter gene, whereas Westerners and members of some African regions are more likely to carry the L allele (Chiao & Blizinksy, 2010; Gelernter, Kranzler, & Cubells, 1997). Paradoxically, despite increased prevalence of the S allele in East Asia, these nations exhibit a decreased prevalence of anxiety and mood disorders, including MDD (Chiao & Blizinsky, 2010; Kessler & Ustun, 2008). This suggests that collectivistic cultures may actually buffer against the onset of emotional disorders characterized by diminished PE—such as MDD—even despite carrying an increased genetic risk for negative emotion responding. Thus, gene-behavior associations may vary cross culturally, indicating the importance of studying the role of culture in gene-by-environment interaction models of mental disorders (e.g., culture-by-gene-by-environment or C × G × E) (Chiao, 2011).
The second genetic polymorphism of interest is a polymorphic region within the exon III region of the dopamine D4 receptor gene (DRD4). Similar to 5-HTTLPR, it is both implicated in affective differences and varies in allelic frequency across cultures (Chiao & Ambady, 2007). The dopaminergic system plays a key role in reward (Spanagel & Weiss, 1999), and thus in PE. The “long” (L) allele of this polymorphic region within the DRD4 gene has been associated with approach-related behaviors, such as the personality trait of novelty-seeking (Ebstein et al., 1996), risky behaviors in laboratory settings (Dreber et al., 2009; Kuhnen & Chiao, 2009), and increased substance craving in addicts (Hutchinson, McGeary, Smolen, Bryan, & Swift, 2002; McClernon, Hutchison, Rose, & Koznick, 2007). Prevalence of the L allele is highly variable cross-culturally, with the lowest prevalence in East Asian populations and highest prevalence in South American Indian populations (Chen, Burton, Greenberger, & Dmitrieva, 1999). Further, Chen and colleagues (1999) note that populations which migrated longer distances or lived nomadic lifestyles in prehistoric times also show significantly greater prevalence of the L allele. The authors propose that personality traits such as novelty-seeking might have been adaptive in such circumstances, leading to genetic selection for this allele. This suggests a particularly important role of this polymorphism in emotional disorders characterized primarily by heightened reward seeking or craving, such as SUDs. As such individuals with the L allele may be at particular risk for the onset and expression of SUDs that reside outside of East Asian cultures. These and other findings surrounding genetic polymorphisms of interest indicate the importance of effects of genes on cultural tendencies, and vice versa, in the etiology of affective disorders. Genetic analysis may therefore be another useful tool in developing a cultural approach to disorders of PER.
Psychophysiology
Though understudied in cultural neuroscience research, psychophysiology methods seek to link peripheral physiological phenomena (e.g., cardiovascular activity) with psychological phenomena (e.g., joy or fear) and compare physiological responses across individuals as they engage in emotion-relevant tasks. One of the most promising psychophysiology markers within the domain of PER, and one that can be expanded to the cultural domain, is respiratory sinus arrhythmia (RSA; Kok & Fredrickson, 2010). RSA measures neural control over the heart rate by means of the vagus nerve (Porges, 1992). When measured at rest, RSA has been shown to be positively associated with measures of PE such as positive mood, extraversion, agreeableness, and optimism (Kok & Fredrickson, 2010; Oveis et al., 2009). Another line of work documents growing associations between RSA reactivity (or changes in resting RSA in response to an external or internal stimulus) with the ability to flexibly regulate one’s emotions (e.g., Butler, Wilhelm, & Gross, 2006). Thus RSA has been putatively considered as a potential physiological marker of both positive affect and regulatory control over one’s emotions.
As previously mentioned, RSA has been observed to be heightened in BD (Gruber et al., 2008; Gruber, Harvey & Purcell, 2011), and in some cases decreased in those with MDD (Beauchaine, 2001). Thus, RSA is a promising physiological marker of disorders characterized by a relative excess or deficiency of PE, as well as the ability to adaptively and flexibly modulate one’s emotions. Of particular importance is the fact that both BD and MDD are marked by troubles with PER, both show dysregulated psychophysiological correlates of PER either by excessively elevated RSA levels in BD or diminished RSA levels in MDD. Although we know that physiological parameters of cardiovascular responding differ cross-culturally, and these same parameters are uniquely associated with both PER and the key emotional disorders discussed, no work to date has systematically examined the possible role of cultural variation. One possibility is that cultures vary in their use of specific kinds of physiological profiles when attempting to regulate PE. This is not surprising given prior research demonstrating the psychophysiological costs of suppressing emotions appear to be reduced in Americans who endorse Asian compared to Western European values (Butler, Lee, & Gross, 2007). This suggests that Asian cultures may show less psychophysiological toll— and perhaps preserved engagement of flexible RSA levels—when engaging in particular types of regulation strategies. As such Asian culture may serve as a buffer against the physiological costs and correlates of emotional disorders such as MDD and BD.
Behavior
Of particular use in developing a cultural approach towards PE and its disorders are two measures that effectively tap into PE regulation and reactivity; namely, self-reported emotion and coding facial expressions of emotional displays.
Self-report methodologies have often been used to assess cultural variation in PE as well as PE regulation. With respect to measuring PE, Positive and Negative Affective Schedule (PANAS) (Watson, Clark, & Tellegen, 1988) measures self-reported positive and negative moods over a specified time frame, while modified versions of the Differential Emotions Scale (DES) (Izard, 1972) measure discrete experiences of PE ranging from high-arousal positive emotions like joy to low-arousal positive emotions like serenity. With respect to PER, several self-report measures have been developed to tap into different ways to regulate emotions more generally and positive emotion specifically. For example, the Emotion Regulation Questionnaire (ERQ) (Gross & John, 2003) measures people’s tendency to reappraise or suppress their emotions and has been utilized in major studies of culture and emotion regulation (Matsumoto, Yoo, Nakagawa, et al., 2008; Miyamoto & Ma, 2011), as well as to study several of our key disorders of PE (e.g., Aldao, Nolen-Hoksema, & Schweizer, 2009). Moreover, recently emerging scales have gained momentum that specifically examine maladaptive forms of regulating positive emotion, such as ruminating over the causes and consequences of one’s positive mood in a way that is not conducive to problem-solving (i.e., positive rumination; Feldman, Joormann, & Johnson, 2008) as well as engaging in maladaptive and impulsive behaviors when in the context of a current positive mood (i.e., positive urgency; Cyders et al., 2007). Importantly both positive rumination and positive urgency have been associated with an increased risk for, and diagnosis of, BD (e.g., Gruber, 2011).
Though self-report serves as a potential starting point for measuring cultural differences, it is rarely a sufficient method on its own. This method is complicated by the fact that researchers must construct culturally-appropriate surveys or tasks when working with less well-studied populations, so may additionally utilize anthropological methods like open-ended interviews (Chiao, Hariri, et al., 2010; Saxbe et al., 2012). (Heine, 2008). Beyond the issue of translation, however, cultural biases exist even in the way that scales are used. For example, people from Western cultures are more likely to use extreme ends of a Likert scale than are those from East Asian cultures (Heine, 2008). For reasons such as this, it is critical to supplement self-report measures with behavioral methods in order to ensure a more objective measurement in the study of emotion. Particularly relevant with respect to PE, facial expression coding systems are one means of gathering unobtrusive measures of emotional displays. With such systems, researchers record facial expressions during an experiment, and then code emotions based on the facial expressions of participants. This allows for the observation of either discrete PE’s, such as enjoyment, pride or amusement (coded with the Emotion Facial Action Coding System; Ekman, Friesen, & Hager, 2002; Ekman & Rosenberg, 1997), or of more global expressions of PE, such as general happiness (e.g., the Emotional Expressive Behavior Coding System, Gross & Levenson, 1993). Given this method’s suitability for use in many cultures, its capacity to capture quick displays as they occur, and its immunity to biases in memory or report, it is an optimal method to include in a cultural approach towards PE and related disorders. Furthermore highlighting the need to study PE displays through a cultural lens, the capacity for prosocial and self-conscious positive emotions, such as pride, compassion and awe, (Tangney & Tracy, 2012) vary across individuals and cultures (Tracy & Matsumoto, 2008). For instance, the behavioral expression of pride is universally perceived and expressed as a signal of success across cultures (Tracy & Matsumoto, 2008); however, when and how displays of pride are perceived varies across ethnicities (Tracy & Robins, 2008), likely due to differences in cultural display norms (Matsumoto, Yoo, Fontaine, et al., 2008). In terms of furthering the cultural study of our four key disorders, facial coding may be especially useful in studying interpersonal processes in SAD; for example, as we reviewed, individuals with SAD express lower levels of PE compared to healthy controls (Turk et al., 2005). Behavioral methods such as these allow for emotional processes to be observed both objectively, and in a social context—both crucial in any cultural study.
Having now introduced cultural neuroscience as a theoretical framework with which to address how culture mediates PER and its related disorders, and having expanded upon the methodological tools that may prove useful to address this, we conclude by discussing theoretical questions that can be used to guide future research endeavors in this area.
Where Do We Go From Here? Concluding Remarks and a Future Roadmap
Thus far we’ve illustrated the central role of PER in psychopathology, discussed the critical influence of culture on PE and psychopathology, and outlined the theoretical framework and methodological tools necessary to begin cultural neuroscience investigations into PER. Given that present literature provides important pieces—but not a full formation—of an integrative model of each of our four disorders, we suggest in this section three guiding conceptual questions to be used in future research meant to bridge this gap. Specifically, we focus on questions of etiology, dynamics, and treatment of key disorders involving PER, and provide specific examples of research that could result from these guiding themes.
Etiology
A first question concerns the origin of our four key disorders: specifically, how can we understand the etiology of PE regulation difficulties within specific cultural contexts? Many conceive of psychopathology as a product of environment and biology, as articulated in gene-by-environment interaction (Caspi et al., 2010) models of disorder. As we have demonstrated, culture actually factors into both ends of this equation, especially with respect to PER, making cultural neuroscience theoretically valuable in explaining related disorders. From a more practical standpoint, formulating more precise models of the cause of our key disorders may allow us, for example, to predict which individuals or groups are most vulnerable to their onset—allowing for preventative efforts targeted towards these individuals.
The particular example of BD—along with our general category of disorders involving heightened PE—helps to illustrate how the question of etiology can guide a program of cultural neuroscience research. Given the evidence so far, we hypothesize that certain genetic phenotypes or neural differences may act as risk factors for BD in some cultural contexts, but not in others. For example, Western cultural values, as we have reviewed, not only specify more extreme positive emotional goals (Tsai et al., 2006), but also encourage the maintenance and up-regulation of these states once they are achieved (Miyamoto & Ma, 2011). Such values may enhance PE regulation difficulties in individuals with BD who are already predisposed, via individual differences in the dopaminergic system, towards increased reward sensitivity and heightened positive emotionality (e.g., Johnson, 2005). Eastern cultural values, which conversely encourage more moderate and low-arousal positive feelings, as well as strategies of down-regulating PE, could potentially provide a protective factor against the onset and exacerbation of symptoms in individuals with BD. Thus, a cultural neuroscience investigation of PER in BD could examine PER in BD members from two distinct cultural groups, looking to find an interaction between cultural and biological influences on these outcomes. A next step would be to investigate, with respect to BD, the downstream consequences of these risky or culturally-buffered dispositions.
With respect to deficits in PE, one potential etiological question concerns the relationship between SAD and a related disorder observed in Japan, Taijin-Kyofu-Sho (TKS), marked by a fear of adversely affecting others in social situations. One question concerning these two disorders is whether the same or different risk factors lead to these two outcomes (Nakamura, Kitanishi, Miyake, Hashimoto, & Kubota, 2002). Here, cultural neuroscience investigations can provide the tools to delve into questions of mechanism surrounding both disorders, sidestepping issues of self-report which may miss their commonalities. For example, coding of facial displays of PE during social interaction, or neural investigations into the mechanisms of social reward in patients with TKS and SAD, could uncover the general or culture-specific mechanisms which cause these two disorders.
Overall, projects motivated by questions of etiology, such as the two lines of research described here, promise to provide overall models of our key disorders. Presently, we find reason to believe that while many of the processes that underlie disorders are universal, culture may at least in theory lead to difference in the very processes that create disorder. Such a hypothesis remains an open question, and will be an important direction for future research.
Cultural Dynamics
A second question concerns shifts in cultural dynamics; specifically, when a person’s cultural context changes, how might PER and related disorders be affected? Given the multiculturalism and increased prevalence of minorities and immigrants within the United States (Pew Research Center for Social and Demographic Trends, 2008; Smelser, Wilson, Mitchell, 2001), and the increasing globalization of the world (Arnett, 2002; Henrich, Heine, Norenzayan, 2008), it is necessary to understand how cultural influences on PER can vary over time, and with changing cultural influences. Indeed, there is strong evidence to believe that emotional processes can in fact change due to shifts in cultural context. For example, when moving from one culture to another, the style (e.g., identity integration or separation) (Berry, 1980) and extent of one’s acculturation predict how similar one’s emotional experience will be to that of the new host culture (Liem, Lim, & Liem, 2000). Thus, we might predict similar patterns with respect to PER, with important practical extensions regarding disorder. Recent cultural neuroscience research has shown that length of stay within a region is inversely correlated with amygdala response to facial emotions (Derntl et al., 2009). Hence, acculturation that occurs across the first five years of living within a region may modulate amygdala response to emotion and may similarly affect PER.
As one example, the study of MDD can profit from a cultural neuroscience investigation of the dynamics of PER. One group reports that greater discrepancies between ideal and actual experienced HAP emotions predict greater depression for American participants, versus ideal-actual LAP discrepancies for Chinese participants, and both types of discrepancies for Asian-Americans (Tsai et al., 2006). One extension of this finding is to explore how depressed individuals originally from an Eastern culture fare with respect to regulation patterns and mental health outcomes as they identify as increasingly more Western. Does aiming for a higher arousal standard help encourage individuals with MDD to maintain PE, or does it exacerbate the problem because of an inability to meet this cultural ideal? More specifically regarding regulation in bicultural individuals, research examining neural activity during PER within regions associated with PE reactivity and regulation can help us infer whether and how regulation is working in groups exposed to multiple ideals regarding appropriate regulatory strategies (i.e., dampening or savoring, which on the surface seem contradictory). Overall, there is room for a large amount of progress in understanding dynamics of PER via cultural neuroscience tools.
Translational application
A third question concerns the ability to translate these findings towards the development of targeted treatment of psychopathology. Specifically, how can we best treat disorders related to PER, working with our knowledge of cultural differences? Because, as we have argued, cultural values are particularly important in determining ideals regarding positive—as opposed to negative—emotional experiences, the issue of culturally sensitive treatment is especially pertinent to our four key disorders. A cultural neuroscience investigation can illuminate culturally-determined barriers to treatment, as well as biological mechanisms for targeted treatment across cultures.
BD represents a disorder with significant treatment barriers related to PER. We know that one reason for medication non-adherence is that patients report “missing highs” (Goodwin & Jamison, 1990), or in other words, not actually wishing to reduce their experienced PE associated with manic episodes. From a cultural perspective, it may be that this reason for non-compliance is exacerbated in the West, but lessened in the East, given that Easterners see reason to, and do, dampen PE. Clinicians in this case can encourage Americans patients, for example, to adopt more moderate ideals or to understand the potentially mixed nature of positive states. If effective, such cognitive changes could aid in treatment compliance. Additionally aiding the development of culturally-suited treatments (and as discussed more thoroughly above in our section on etiology), cultural neuroscience is well-suited to uncover both biological and cultural origins of disorder, as well as their relative influences and potential interactions. Making use of these findings, clinicians will better understand when different treatments, e.g., cognitive approaches versus psychotropic medications, are most useful.
Concluding Remarks
Taken together, these three broad themes help generate novel ideas for cultural neuroscience investigations of PER. In summary, we propose a cultural neuroscience approach to address how cultural factors influence PE regulation and related mental health disorders. We have outlined a roadmap of potential themes to explore in this domain, as well as the cultural neuroscience tools with which to explore them. Through this approach, we hope to develop a more comprehensive understanding of the basic mechanisms of human emotion, which can be translated to identify the behavioral endophenotypes that confer risk for disorder. Ultimate goals of this approach are improving targeted treatments that are responsive to the sociocultural contexts of diverse patient populations, and closing the gap in population mental health disparities. Finally, as we conclude below, we believe that research into the etiology, cultural dynamics, and translational applications towards developing targeted treatments for positive emotion regulation difficulties may ultimately lead to reducing global mental health disparities.
Footnotes
Given that emotion regulation is a broad concept (Gross & Thompson, 2007), many studies note the difficulty of disentangling it from emotion reactivity, defined as a change in emotion responding from one’s baseline (Silvers et al., 2012; McRae et al., 2008). Indeed, due to the speed with which individuals automatically and unconsciously regulate their emotions (Mauss, Bunge & Gross, 2007), it may be that most research on emotion reactivity inherently includes some component of emotion regulation. Given this blurry distinction, and in order to more comprehensively understand the complexities of PER and psychopathology, we focus primarily on emotion regulation literature but address literature on emotion reactivity as it is relevant. We also note that although negative emotion regulation is an equally important domain for future inquiry, it is beyond the scope of the present chapter.
The present review has not discussed at length the interpersonal social processes which contribute to disorder, but here the transdiagnostic perspective lends important insight as well. In particular, a host of negative social processes—for example, expressions of hostility, criticism, and emotional over-involvement by family members (Brown, Birley, & Wing, 1972),Please add to reference list and excessively controlling parenting styles (Chorpita & Barlow, 1998)—have been shown to be transdiagnostic (e.g Wearden, Tarrier, Barrowclough, Zastowny, & Rahill, 2000) Please add to ref list. This point further highlights an unexplored aspect of the disorders we have presently discussed; namely, that a host of negative, and likely transdiagnostic processes—whether emotional (e.g., Kring, 2008) or social—also play a role in the development of psychopathology. Such a discussion is beyond the scope of the present paper, but represents an important future direction for a cultural neuroscience investigation of psychopathology.
References
- Aguilar de Arcos F, Verdejo-Garcia A, Peralta-Ramirez MI, Sanchez-Barrera M, Perez-Garcia M. Drug and Alcohol Dependence. 2005;78:159–167. doi: 10.1016/j.drugalcdep.2004.10.010. http://dx.doi.org/10.1016/j.drugalcdep.2004.10.010 [DOI] [PubMed] [Google Scholar]
- Aldao A, Nolen-Hoeksema S, Scheweizer S. Emotion-regulation strategies across psychopathology: a meta-analytic review. Clinical Psychology Review. 2009;30:217–237. doi: 10.1016/j.cpr.2009.11.004. http://dx.doi.org/10.1016/j.cpr.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Allen NB, Trinder J, Brennan C. Affective startle modulation in clinical depression: Preliminary findings. Biological Psychiatry. 1999;46:542–550. doi: 10.1016/s0006-3223(99)00025-6. http://dx.doi.org/10.1016/S0006-3223(99)00025-6. [DOI] [PubMed] [Google Scholar]
- Almeida JRC, Versace A, Hassel S, Kupfer DJ, Phillips ML. Elevated amygdala activity to sad facial expressions: a state marker of bipolar but not unipolar depression. Biological Psychiatry. 2010;67:414–421. doi: 10.1016/j.biopsych.2009.09.027. http://dx.doi.org/10.1016/j.biopsych.2009.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambady N, Bharucha J. Culture and the brain. Current Directions in Psychological Science. 2009;18:342–345. http://dx.doi.org/10.1111/j.1467-8721.2009.01664.x. [Google Scholar]
- Arnett JJ. The psychology of globalization. American Psychologist. 2002;57:774–783. doi: 10.1037/0003-066X.57.10.774. http://dx.doi.org/10.1037/0003-066X.57.10.774. [DOI] [PubMed] [Google Scholar]
- Alonso J, Vilagut G, Chatterji S, Heeringa S, Schoenbaum M, Ustun TB, Kessler RC. Including information about co-morbidity in estimates of disease burden: results from the World Health Organization world mental health surveys. Psychological Medicine. 2011;41:873–886. doi: 10.1017/S0033291710001212. http://dx.doi.org/10.1017/S0033291710001212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV-TR. Washington, DC: American Psychiatric Association; 2000. Task Force on DSM-IV. [Google Scholar]
- Asnaani A, Richey JA, Dimaite R, Hinton DE, Hofmann SG. A Cross-Ethnic Comparison of Lifetime Prevalence Rates of Anxiety Disorders. Journal of Nervous and Mental Disorders. 2010;198(8):551–555. doi: 10.1097/NMD.0b013e3181ea169f. http://dx.doi.org/10.1097/NMD.0b013e3181ea169f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine T. Vagal tone, development, and Gray’s motivational theory: Toward an integrated model of autonomic nervous system functioning in psychopathology. Development and Psychopathology. 2001;13:183–214. doi: 10.1017/s0954579401002012. http://dx.doi.org/10.1017/S0954579401002012. [DOI] [PubMed] [Google Scholar]
- Beck A, Schlangenhauf F, Wustenberg T, Hein J, Kienast T, Kahnt T, Wrase J. Biological Psychiatry. 2009;66:734–742. doi: 10.1016/j.biopsych.2009.04.035. http://dx.doi.org/10.1016/j.biopsych.2009.04.035. [DOI] [PubMed] [Google Scholar]
- Berenbaum H, Oltmanns TF. Emotional experience and expression in schizophrenia and depression. Journal of Abnormal Psychology. 1992;101:37–44. doi: 10.1037//0021-843x.101.1.37. http://dx.doi.org/10.1037/0021-843X.101.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Kringelbach ML. Affective neuroscience of pleasure: reward in humans and animals. Psychopharmacology. 2008;199:457–480. doi: 10.1007/s00213-008-1099-6. http://dx.doi.org/10.1007/s00213-008-1099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry JW. Acculturation as varieties of adaptation. In: Padilla A, editor. Acculturation: Theory, models and findings. Boulder, CO: Westview; 1980. pp. 9–25. [Google Scholar]
- Brislin RW. Back-translation for cross-cultural research. Journal of Cross-Cultural Psychology. 1970;1:185–121. http://dx.doi.org/10.1177/135910457000100301. [Google Scholar]
- Boyd R, Richerson PJ. Why is culture adaptive? The Quarterly Review of Biology. 1983;58:209–214. http://dx.doi.org/10.1086/413217. [Google Scholar]
- Brown GW, Birley JLT, Wing JK. The influence of family life on the course of schizophrenic disorders: A replication. British Journal of Psychiatry. 1972;121:241–258. doi: 10.1192/bjp.121.3.241. http://dx.doi.org/10.1192/bjp.121.3.241. [DOI] [PubMed] [Google Scholar]
- Brown TA, Chorpita BF, Barlow DH. Structural relationships among dimensions of the DSM-IV anxiety and mood disorders and dimensions of negative affect, positive affect, and autonomic arousal. Journal of Abnormal Psychology. 1998;107:179–192. doi: 10.1037//0021-843x.107.2.179. http://dx.doi.org/10.1037/0021-843X.107.2.179. [DOI] [PubMed] [Google Scholar]
- Brown SM, Hariri AR. Neuroimaging studies of serotonin gene polymorphisms: Exploring the interplay of genes, brain, and behavior. Cognitive, Affective, & Behavioral Neuroscience. 2006;6:44–52. doi: 10.3758/cabn.6.1.44. http://dx.doi.org/10.3758/CABN.6.1.44. [DOI] [PubMed] [Google Scholar]
- Burns AB, Brown JS, Sachs-Ericsson N, Plant EA, Curtis JT, Fredrickson BL, Joiner TE. Upward spirals of positive emotion and coping: Replication, extension, and initial exploration of neurochemical substrates. Personality and Individual Differences. 2008;44:360–370. doi: 10.1016/j.paid.2007.08.015. http://dx.doi.org/10.1016/j.paid.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler EA, Wilhelm FH, Gross JJ. Respiratory sinus arrhythmia, emotion, and emotion regulation during social interaction. Psychophysiology. 2006;43:612–622. doi: 10.1111/j.1469-8986.2006.00467.x. http://dx.doi.org/10.1111/j.1469-8986.2006.00467.x. [DOI] [PubMed] [Google Scholar]
- Butler EA, Lee TL, Gross JJ. Emotion regulation and culture: Are the social consequences of emotion suppression culture-specific? Emotion. 2007;7:30–48. doi: 10.1037/1528-3542.7.1.30. http://dx.doi.org/10.1037/1528-3542.7.1.30. [DOI] [PubMed] [Google Scholar]
- Bylsma LM, Morris BH, Rottenberg J. A meta-analysis of emotional reactivity in major depressive disorder. Clinical Psychology Review. 2008;28:676–691. doi: 10.1016/j.cpr.2007.10.001. http://dx.doi.org/10.1016/j.cpr.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, Poulton R. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. http://dx.doi.org/10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Caspi A, Hariri AR, Holmes A, Uher R, Moffitt TE. Genetic sensitivity to the environment: The case of the serotonin transporter gene and its implications for studying complex diseases and traits. American Journal of Psychiatry. 2010;167:509–527. doi: 10.1176/appi.ajp.2010.09101452. http://dx.doi.org/10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheetham A, Allen NB, Yucel M, Lubman DI. The role of affective dysregulation in drug addiction. Clinical Psychology Review. 2010;30:621–634. doi: 10.1016/j.cpr.2010.04.005. http://dx.doi.org/10.1016/j.cpr.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Chen CS, Burton M, Greenberger E, Dmitrieva J. Population migration and the variation of dopamine D4 receptor (DRD4) allele frequencies around the globe. Evolution and Human Behavior. 1999;20:309–324. http://dx.doi.org/10.1016/S1090-5138(99)00015-X. [Google Scholar]
- Chen C, Wong J, Lee N, Chan-Ho M, Lau JT, Fung M. The Shatin community mental health survey in Hong Kong. Archives of General Psychiatry. 1993;50:125–133. doi: 10.1001/archpsyc.1993.01820140051005. http://dx.doi.org/10.1001/archpsyc.1993.01820140051005. [DOI] [PubMed] [Google Scholar]
- Cheon BK, Chiao JY. Cultural variations in mental illness stigma. Journal of Cross-Cultural Psychology. doi: 10.1177/0022022112455457. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiao JY. Cultural neuroscience: Visualizing culture-gene influences on brain function. In: Decety J, Cacioppo J, editors. The Oxford handbook of social neuroscience. Oxford: Oxford University Press; 2011. pp. 742–762. http://dx.doi.org/10.1093/oxfordhb/9780195342161.013.0049. [Google Scholar]
- Chiao JY, Ambady N. Cultural neuroscience: Parsing universality and diversity across levels of analysis. In: Kitayama S, Cohen D, editors. Handbook of cultural psychology. New York, NY: Guilford Press; 2007. pp. 237–254. [Google Scholar]
- Chiao JY, Blizinsky KD. Culture-gene coevolution of individualism-collectivism and the serotonin transporter gene (5-HTTLPR) Proceedings of the Royal Society B: Biological Sciences. 2010;277:529–37. doi: 10.1098/rspb.2009.1650. http://dx.doi.org/10.1098/rspb.2009.1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiao JY, Blizinsky KD. Population disparities in mental health: Insights from cultural neuroscience. American Journal of Public Health. doi: 10.2105/AJPH.2013.301440. (under review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiao JY, Cheon BK. The weirdest brains in the world. Behavioral and Brain Sciences. 2010;33:28–30. doi: 10.1017/S0140525X10000282. http://dx.doi.org/10.1017/S0140525×10000282. [DOI] [PubMed] [Google Scholar]
- Chiao JY, Harada T, Komeda H, Li Z, Mano Y, Saito DN, Iidaka T. Dynamic cultural influences on neural representations of the self. Journal of Cognitive Neuroscience. 2010;22:1–11. doi: 10.1162/jocn.2009.21192. http://dx.doi.org/10.1162/jocn.2009.21192. [DOI] [PubMed] [Google Scholar]
- Chiao JY, Harada T, Komeda H, Li Z, Mano Y, Saito DN, Iidaka T. Neural basis of individualistic and collectivistic views of self. Human Brain Mapping. 2009;30:2813–2820. doi: 10.1002/hbm.20707. http://dx.doi.org/10.1002/hbm.20707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiao JY, Hariri AR, Harada T, Mano Y, Sadato N, Parrish TB, Iidaka T. Theory and methods in cultural neuroscience. Social Cognitive and Affective Neuroscience. 2010;5:356–361. doi: 10.1093/scan/nsq063. http://dx.doi.org/10.1093/scan/nsq063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiao JY, Immordino-Yang MH. Modularity and the cultural mind: Contributions of cultural neuroscience to cognitive theory. Perspectives on Psychological Science. doi: 10.1177/1745691612469032. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chorpita BF, Barlow DH. The development of anxiety: The role of control in the early environment. Psychological Bulletin. 1998;124:3–21. doi: 10.1037/0033-2909.124.1.3. http://dx.doi.org/10.1037/0033-2909.124.1.3. [DOI] [PubMed] [Google Scholar]
- Clark LA, Watson D, Mineka S. Temperament, personality, and the mood and anxiety disorders. Journal of Abnormal Psychology. 1994;103:103–116. http://dx.doi.org/10.1037/0021-843X.103.1.103. [PubMed] [Google Scholar]
- Collins PY, Patel V, Joestl SS, March D, Insel TR, Daar AS. Grand challenges in global mental health. Nature. 2011;475:27–30. doi: 10.1038/475027a. http://dx.doi.org/10.1038/475027a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyders MA, Smith GT, Spillane NS, Fischer S, Annus AM, Peterson C. Integration of impulsivity and positive mood to predict risky behavior: Development and validation of a measure of positive urgency. Psychological Assessment. 2007;19:107–118. doi: 10.1037/1040-3590.19.1.107. http://dx.doi.org/10.1037/1040-3590.19.1.107. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Abercrombie H, Nitschke JB, Putnam K. Regional brain function, emotion, and disorders of emotion. Current Opinion in Neurobiology. 1999;9:228–234. doi: 10.1016/s0959-4388(99)80032-4. http://dx.doi.org/10.1016/S0959-4388(99)80032-4. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Pizzagalli D, Nitschke JB, Putnam K. Depression: Perspectives from affective neuroscience. Annual Review of Psychology. 2002;53:545–574. doi: 10.1146/annurev.psych.53.100901.135148. http://dx.doi.org/10.1146/annurev.psych.53.100901.135148. [DOI] [PubMed] [Google Scholar]
- Derntl B, Haber U, Robinson S, Windischberger C, Kryspin-Exner I, Gur RC, Moser E. Amygdala activation during recognition of emotions in a foreign ethnic group is associated with duration of stay. Social Neuroscience. 2009;4:294–307. doi: 10.1080/17470910802571633. http://dx.doi.org/10.1080/17470910802571633. [DOI] [PubMed] [Google Scholar]
- Dettling M, Heinz A, Dufeu P, Rommelspacher H, Graf K, Schmidt LG. Dopaminergic responsivity in alcoholism: Trait, state, or residual marker? American Journal of Psychiatry. 1995;152:1317–1321. doi: 10.1176/ajp.152.9.1317. [DOI] [PubMed] [Google Scholar]
- Dillon DG, Pizzagalli DA. Maximizing positive emotions: A translational, transdiagnostic look at positive emotion regulation. In: Kring A, Sloan D, editors. Emotion Regulation and Psychopathology: A Transdiagnostic Approach to Etiology and Treatment. New York: The Guilford Press; 2009. [Google Scholar]
- Dowd EC, Barch DM. Pavlovian reward prediction and receipt in schizophrenia: Relationship to anhedonia. Public Library of Science ONE. 2012;7:e35622. doi: 10.1371/journal.pone.0035622. http://dx.doi.org/10.1371/journal.pone.0035622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreber A, Apicella CL, Eisenberg DTA, Garcia JR, Zamore R, Lum JK, Campbell BC. The 7R polymorphism in the dopamine receptor D4 gene (DRD4) is associated with financial risk-taking in men. Evolution and Human Behavior. 2009;30:85–92. http://dx.doi.org/10.1016/j.evolhumbehav.2008.11.001. [Google Scholar]
- Dressler WW, Balieiro MC, Ribeiro RP, Dos Santos JE. Cultural consonance, a 5HT2A receptor polymorphism, and depressive symptoms: A longitudinal study of gene × culture interaction in urban Brazil. American Journal of Human Biology. 2009;21:91–97. doi: 10.1002/ajhb.20823. http://dx.doi.org/10.1002/ajhb.20823. [DOI] [PubMed] [Google Scholar]
- Dunn BD, Dalgleish T, Lawrence AD, Cusack R, Ogilvie AD. Categorical and dimensional reports of experienced affect to emotion-inducing pictures in depression. Journal of Abnormal Psychology. 2004;113:654–660. doi: 10.1037/0021-843X.113.4.654. http://dx.doi.org/10.1037/0021-843X.113.4.654. [DOI] [PubMed] [Google Scholar]
- Ebstein RP, Novick O, Umansky R, Priel B, Osher Y, Blaine D, Belmaker RH. Dopamine D4 receptor (D4DR) exon III polymorphism associated with the human personality train of novelty seeking. Nature Genetics. 1996;12:78–80. doi: 10.1038/ng0196-78. http://dx.doi.org/10.1038/ng0196-78. [DOI] [PubMed] [Google Scholar]
- Ekman P, Friesen WV, Hager JC. Facial action coding system. 2nd ed. London: Weidenfeld & Nicolson; 2002. [Google Scholar]
- Ekman P, Rosenberg EL. What the face reveals: Basic and applied studies of spontaneous expression using the facial action coding system (FACS) New York: Oxford University Press; 1997. [Google Scholar]
- Elliott R, Ogilvie A, Rubinsztein JS, Calderon G, Dolan RJ, Sahakian BJ. Abnormal ventral frontal response during performance of an affective go/no go task in patients with mania. Biological Psychiatry. 2004;55:1163–1170. doi: 10.1016/j.biopsych.2004.03.007. http://dx.doi.org/10.1016/j.biopsych.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Farmer A, Lam D, Sahakian B, Roiser J, Burke A, O’Neill N, McGuffin P. A pilot study of positive mood induction in inter-episode bipolar subjects compared with healthy controls. Psychological Medicine. 2006;36:1213–1218. doi: 10.1017/S0033291706007835. http://dx.doi.org/10.1017/S0033291706007835. [DOI] [PubMed] [Google Scholar]
- Feldman GC, Joormann J, Johnson SL. Responses to positive affect: A self-report measure of rumination and dampening. Cognitive Therapy and Research. 2008;32:507–525. doi: 10.1007/s10608-006-9083-0. http://dx.doi.org/10.1007/s10608-006-9083-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Dahl RE. Neural systems of positive affect: Relevance to understanding child and adolescent depression? Development and Psychopathology. 2005;17:827–850. doi: 10.1017/S095457940505039X. http://dx.doi.org/10.1017/S095457940505039X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredickson BL. What good are positive emotions? Review of General Psychology. 1998;2:300–319. doi: 10.1037/1089-2680.2.3.300. http://dx.doi.org/10.1037/1089-2680.2.3.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber J, Braafladt N, Weiss B. Affect regulation in depressed and nondepressed children and young adolescents. Development and Psychopathology. 1995;7:93–115. http://dx.doi.org/10.1017/S0954579400006362. [Google Scholar]
- Garnefski N, Kraaij V. Relationships between cognitive emotion regulation strategies and depressive symptoms: A comparative study of five specific samples. Personality and Individual Differences. 2006;40:1659–1669. http://dx.doi.org/10.1016/j.paid.2005.12.009. [Google Scholar]
- Gelernter J, Kranzler H, Cubells JF. Serotonin transporter protein (SLC6A4) allele and haplotype frencies and linkage disequilibria in African- and European-American and Japanese populations and in alcohol-dependent subjects. Human Genetics. 1997;101:243–246. doi: 10.1007/s004390050624. http://dx.doi.org/10.1007/s004390050624. [DOI] [PubMed] [Google Scholar]
- Gessa GL, Muntoni F, Collu M, Vargui L, Mereu G. Low doses of ethanol activate dopaminergic neurons in the ventral tegmental area. Brain Research. 1985;348:201–203. doi: 10.1016/0006-8993(85)90381-6. http://dx.doi.org/10.1016/0006-8993(85)90381-6. [DOI] [PubMed] [Google Scholar]
- Gilman JM, Ramchandani VA, Davis MB, Bjork JM, Hommer DW. Why we like to drink: A functional magnetic resonance imaging study of rewarding and anxiolytic effects of alcohol. Journal of Neuroscience. 2008;28:4853–4591. doi: 10.1523/JNEUROSCI.0086-08.2008. http://dx.doi.org/10.1523/JNEUROSCI.0086-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin F, Jamison JK. Manic depressive illness. New York, NY: Oxford University Press; 1990. [Google Scholar]
- Green MJ, Cahill CM, Malhi GS. The cognitive and neurophsyiological dysregulation in bipolar disorder. Journal of Affective Disorders. 2007;103:29–42. doi: 10.1016/j.jad.2007.01.024. http://dx.doi.org/10.1016/j.jad.2007.01.024. [DOI] [PubMed] [Google Scholar]
- Gross JJ, John OP. Individual differences in two emotion regulation processes: Implications for affect, relationships, and well-being. Journal of Personality and Social Psychology. 2003;85:348–362. doi: 10.1037/0022-3514.85.2.348. http://dx.doi.org/10.1037/0022-3514.85.2.348. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Levenson RW. Emotional suppression: Physiology, self-report, and expressive behavior. Journal of Personality and Social Psychology. 1993;64:970–986. doi: 10.1037//0022-3514.64.6.970. http://dx.doi.org/10.1037/0022-3514.64.6.970. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Thompson RA. Emotion regulation: Conceptual foundations. In: Gross J, editor. Handbook of emotion regulation. New York, NY: Guilford Press; 2007. pp. 3–24. [Google Scholar]
- Gruber J. When feeling good can be bad: Positive emotion persistence (PEP) in bipolar disorder. Current Perspectives in Psychological Science. 2011;20:217–221. http://dx.doi.org/10.1177/0963721411414632. [Google Scholar]
- Gruber J, Culver JL, Johnson SL, Nam J, Keller KL, Ketter TK. Do positive emotions predict symptomatic change in bipolar disorder? Bipolar Disorders. 2009;11:330–336. doi: 10.1111/j.1399-5618.2009.00679.x. http://dx.doi.org/10.1111/j.1399-5618.2009.00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber J, Eidelman P, Harvey AG. Transdiagnostic emotion regulation processes in bipolar disorder and insomnia. Behaviour Research and Therapy. 2008;46:1096–1100. doi: 10.1016/j.brat.2008.05.004. http://dx.doi.org/10.1016/j.brat.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Gruber J, Eidelman P, Johnson SL, Smith B, Harvey AG. Hooked on a feeling: Rumination about positive and negative emotion in inter-episode bipolar disorder. Journal of Abnormal Psychology. doi: 10.1037/a0023667. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber J, Harvey AG, Purcell AL. What goes up can come down? A preliminary investigation of emotion reactivity and emotion recovery in bipolar disorder. Journal of Affective Disorders. 2011;133:457–466. doi: 10.1016/j.jad.2011.05.009. http://dx.doi.org/10.1016/j.jad.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber J, Johnson SL, Oveis C, Keltner D. Risk for mania and positive emotional responding: Too much of a good thing? Emotion. 2008;8:23–33. doi: 10.1037/1528-3542.8.1.23. http://dx.doi.org/10.1037/1528-3542.8.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber J, Keltner D. “Emotional behavior and psychopathology: A survey of methods and concepts.”. In: Rottenberg J, Johnson S, editors. Emotion and Psychopathology: Bridging Affective and Clinical Science. Washington, D.C: American Psychological Association (APA) Books; 2007. pp. 32–52. [Google Scholar]
- Gruber J, Mauss IB, Tamir M. A dark side of happiness? How, when and why happiness is not always good. Perspectives on Psychological Science. 2011;6:222–233. doi: 10.1177/1745691611406927. http://dx.doi.org/10.1177/1745691611406927. [DOI] [PubMed] [Google Scholar]
- Han S, Northoff G. Culture-sensitive neural substrates of human cognition: a transcultural neuroimaging approach. Nature Reviews Neuroscience. 2008;9:646–654. doi: 10.1038/nrn2456. http://dx.doi.org/10.1038/nrn2456. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, Weinberger DR. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. http://dx.doi.org/10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Weinberger DR. Functional neuroimaging of genetic variation in serotonergic neurotransmission. Genes, Brain and Behavior. 2003;2:341–349. doi: 10.1046/j.1601-1848.2003.00048.x. http://dx.doi.org/10.1046/j.1601-1848.2003.00048.x. [DOI] [PubMed] [Google Scholar]
- Harvey AG, Watkins ER, Mansell W, Shafran R. Cognitive behavioural processes across psychological disorders: A transdiagnostic approach to research and treatment. Oxford: Oxford University Press; 2004. [Google Scholar]
- Heine SJ. Cultural Psychology. First Edition. New York: Norton; 2008. [Google Scholar]
- Heller AS, Johnstone T, Shackman AJ, Light SN, Peterson MJ, Kolden GG, Davidson RJ. Reduced capacity to sustain positive emotion in major depression reflects diminished maintenance of fronto-striatal brain activation. Proceedings of the National Academy of Sciences. 2009;106:22445–22450. doi: 10.1073/pnas.0910651106. http://dx.doi.org/10.1073/pnas.0910651106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrich J, Heine SJ, Norenzayan A. The weirdest people in the world? Behavioral and Brain Sciences. 2010;33:61–135. doi: 10.1017/S0140525X0999152X. http://dx.doi.org/10.1017/S0140525×0999152X. [DOI] [PubMed] [Google Scholar]
- Herbert JD, Rheingold AA, Brandsma LL. Assessment of social anxiety and social phobia. In: Hofmann S, Dibartolo P, editors. Social anxiety: Clinical, developmental, and social perspectives. London: Elsevier; 2010. pp. 23–62. [Google Scholar]
- Hofmann BU, Meyer TD. Mood fluctuations in people putatively at risk for bipolar disorders. British Journal of Clinical Psychology. 2006;45:105–110. doi: 10.1348/014466505X35317. http://dx.doi.org/10.1348/014466505×35317. [DOI] [PubMed] [Google Scholar]
- Hutchison KE, McGeary J, Smolen A, Bryan A, Swift RM. The DRD4 VNTR polymorphism moderates craving after alcohol consumption. Health Psychology. 2002;21:139–146. http://dx.doi.org/10.1037/0278-6133.21.2.139. [PubMed] [Google Scholar]
- Izard CE. Patterns of emotion: A new analysis of anxiety and depression. New York: Academic Press; 1972. [Google Scholar]
- Johnson SL. Mania and dysregulation in goal pursuit. Clinical Psychology Review. 2005;25:241–262. doi: 10.1016/j.cpr.2004.11.002. http://dx.doi.org/10.1016/j.cpr.2004.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Gruber J, Eisner L. Emotion in bipolar disorder. In: Rottenberg J, Johnson SL, editors. Emotion and psychopathology: Bridging affective and clinical science. Washington, DC: American Psychological Association; 2007. pp. 123–150. http://dx.doi.org/10.1037/11562-006. [Google Scholar]
- Joorman J, Siemer M, Gotlib IH. Mood regulation in depression: Differential effects of distraction and recall of happy memories on sad mood. Journal of Abnormal Psychology. 2007;116:484–490. doi: 10.1037/0021-843X.116.3.484. http://dx.doi.org/10.1037/0021-843X.116.3.484. [DOI] [PubMed] [Google Scholar]
- Kashdan TB, Collins RL. Social anxiety and the experience of positive emotion and anger in everyday life: An ecological momentary assessment approach. Anxiety, Stress, & Coping. 2010;23:259–272. doi: 10.1080/10615800802641950. http://dx.doi.org/10.1080/10615800802641950. [DOI] [PubMed] [Google Scholar]
- Kashdan TB, Roberts JE. Social anxiety, depressive symptoms, and post-event rumination: Affective consequences and social contextual influences. Journal of Anxiety Disorders. 2007;21:284–301. doi: 10.1016/j.janxdis.2006.05.009. http://dx.doi.org/10.1016/j.janxdis.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Kashdan TB, Steger M. Expanding the topography of social anxiety: An experience sampling assessment of positive emotion and events, and emotion suppression. Psychological Science. 2006;17:120–128. doi: 10.1111/j.1467-9280.2006.01674.x. http://dx.doi.org/10.1111/j.1467-9280.2006.01674.x. [DOI] [PubMed] [Google Scholar]
- Kashdan TB, Weeks JW, Savostyanova AA. Whether, how, and when social anxiety shapes positive experiences and events: A self-regulatory framework and treatment implications. Clinical Psychology Review. 2011;31:786–799. doi: 10.1016/j.cpr.2011.03.012. http://dx.doi.org/10.1016/j.cpr.2011.03.012. [DOI] [PubMed] [Google Scholar]
- Kawakami N, Shimizu H, Haratani T, Iwata N, Kitamura T. Lifetime and 6-month prevalence of DSM-III-R psychiatric disorders in an urban community in Japan. Psychiatry Research. 2004;121:293–301. doi: 10.1016/s0165-1781(03)00239-7. http://dx.doi.org/10.1016/S0165-1781(03)00239-7. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Ustun TB. The WHO mental health survey: Global perspectives on the epidemiology of mental disorders. New York, NY: Cambridge University Press; 2008. [Google Scholar]
- Kitayama S, Markus HR, Matsumoto H, Norasakkunkit V. Individual and collective processes in the construction of the self: Self-enhancement in the United States and self-criticism in Japan. Journal of Personality and Social Psychology. 1997;72:1245–1267. doi: 10.1037//0022-3514.72.6.1245. http://dx.doi.org/10.1037/0022-3514.72.6.1245. [DOI] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Adams CM, Varner JL, Hommer D. Dissociation of reward anticipation and outcome with event-related fMRI. NeuroReport. 2001;12:3683–3687. doi: 10.1097/00001756-200112040-00016. http://dx.doi.org/10.1097/00001756-200112040-00016. [DOI] [PubMed] [Google Scholar]
- Kok BE, Fredrickson BL. Upward spirals of the heart: Autonomic flexibility, as indexed by vagal tone, reciprocally and prospectively predicts positive emotions and social connectedness. Biological Psychology. 2010;85:432–436. doi: 10.1016/j.biopsycho.2010.09.005. http://dx.doi.org/10.1016/j.biopsycho.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: Hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. http://dx.doi.org/10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Kring AM. Emotion disturbances as transdiagnostic processes in psychopathology. In: Lewis M, Haviland-Jones J, Barrett LF, editors. Handbook of Emotion. 3rd ed. New York, NY: Guilford Press; 2008. pp. 691–705. [Google Scholar]
- Kring AM, Sloan DS. Emotion regulation and psychopathology: A transdiagnostic approach to etiology and treatment. New York: Guilford Press; 2009. [Google Scholar]
- Kringelbach ML. The human orbitofrontal cortex: Linking reward to hedonic experience. Nature Reviews Neuroscience. 2005;6:691–702. doi: 10.1038/nrn1747. http://dx.doi.org/10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- Kuhnen CM, Chiao JY. Genetic determinants of financial risk taking. Public Library of Science ONE. 2009;4:e4362. doi: 10.1371/journal.pone.0004362. http://dx.doi.org/10.1371/journal.pone.0004362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence NS, Williams AM, Surguladze S, Giampietro V, Brammer MJ, Andrew C, Phillips ML. Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biological Psychiatry. 2004;15:578–587. doi: 10.1016/j.biopsych.2003.11.017. http://dx.doi.org/10.1016/j.biopsych.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Lee CK, Kwak YS, Yamamoto J, Rhee H, Kim YS, Han JH, Choi JO, Lee YH. Psychiatric epidemiology in Korea, Part II: Urban and rural differences. Journal of Nervous and Mental Disease. 1990;178:247–252. doi: 10.1097/00005053-199004000-00005. http://dx.doi.org/10.1097/00005053-199004000-00005. [DOI] [PubMed] [Google Scholar]
- Lee MR, Okazaki S, Yoo HC. Frequency and intensity of social anxiety in Asian Americans and European Americans. Cultural Diversity and Ethnic Minority Psychology. 2006;12:291–305. doi: 10.1037/1099-9809.12.2.291. http://dx.doi.org/10.1037/1099-9809.12.2.291. [DOI] [PubMed] [Google Scholar]
- Leu J, Wang J, Koo K. Are positive emotions just as “positive” across cultures? Emotion. 2011;4:994–999. doi: 10.1037/a0021332. http://dx.doi.org/10.1037/a0021332. [DOI] [PubMed] [Google Scholar]
- Leventhal AM, Kahler CW, Ray LA, Young D, Chelminsksi I, Zimmerman M. Anhedonia and amotivation in psychiatric outpatients with fully remitted stimulant use disorder. American Journal on Addictions. 2008;17:218–223. doi: 10.1080/10550490802019774. http://dx.doi.org/10.1080/10550490802019774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SC. Biocultural orchestration of developmental plasticity across levels: The interplay of biology and culture in shaping the mind and behavioral across the life span. Psychological Bulletin. 2003;129:171–794. doi: 10.1037/0033-2909.129.2.171. http://dx.doi.org/10.1037/0033-2909.129.2.171. [DOI] [PubMed] [Google Scholar]
- Lieber E, Weisner TS. Meeting the practical challenges of mixed methods research. In: Tashakkori A, Teddlie C, editors. Handbook of mixed methods mesearch. Thousand Oaks, CA: Sage; 2010. pp. 559–579. [Google Scholar]
- Liem R, Lim BA, Liem JH. Acculturation and emotion among Asian Americans. Cultural Diversity and Ethnic Minority Psychology. 2000;6:12–31. doi: 10.1037/1099-9809.6.1.13. http://dx.doi.org/10.1037/1099-9809.6.1.13. [DOI] [PubMed] [Google Scholar]
- Lovejoy MC, Steuerwald BL. Subsyndromal unipolar and bipolar disorders: Comparisons on positive and negative affect. Journal of Abnormal Psychology. 1995;104:381–384. doi: 10.1037//0021-843x.104.2.381. http://dx.doi.org/10.1037/0021-843X.104.2.381. [DOI] [PubMed] [Google Scholar]
- Matsumoto D, Yoo SH, Fontaine J, Anguas-Wong AM, Arriola M, Ataca B, Zengeya A. Mapping expressive differences around the world: The relationship between emotional display rules and individualism versus collectivism. Journal of Cross-Cultural Psychology. 2008;39:55–74. http://dx.doi.org/10.1177/0022022107311854. [Google Scholar]
- Matsumoto D, Yoo SH, Nakagawa S, Alexandre J, Altarriba J, Anguas-Wong AM, Zengeya A. Culture, emotion regulation, and adjustment. Journal of Personality and Social Psychology. 2008;94:925–937. doi: 10.1037/0022-3514.94.6.925. http://dx.doi.org/10.1037/0022-3514.94.6.925. [DOI] [PubMed] [Google Scholar]
- Mauss IB, Bunge SA, Gross JJ. Automatic emotion regulation. Social and Personality Psychology Compass. 2007;1:146–167. http://dx.doi.org/10.1111/j.1751-9004.2007.00005.x. [Google Scholar]
- M’Bailara KM, Demotes-Mainard J, Swendsen J, Mathieu F, Leboyer M, Henry C. Emotional hyper-reactivity in normothymic bipolar patients. Bipolar Disorders. 2009;11:63–69. doi: 10.1111/j.1399-5618.2008.00656.x. http://dx.doi.org/10.1111/j.1399-5618.2008.00656.x. [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Hutchison KE, Rose JE, Kozink RV. DRD4 VNTR polymorphism is associated with transient fMRI-BOLD responses to smoking cues. Psychopharmacology. 2007;194:433–441. doi: 10.1007/s00213-007-0860-6. http://dx.doi.org/10.1007/s00213-007-0860-6. [DOI] [PubMed] [Google Scholar]
- McMakin DL, Santiago CD, Shirk SR. The time course of positive and negative emotion in dysphoria. The Journal of Positive Psychology. 2009;4:182–192. doi: 10.1080/17439760802650600. http://dx.doi.org/10.1080/17439760802650600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae K, Ochsner KN, Mauss IB, Gabrieli JDE, Gross JJ. Gender differences in emotion regulation: An fMRI study of cognitive reappraisal. Group Processes and Intergroup Relations. 2008;11:145–162. doi: 10.1177/1368430207088035. http://dx.doi.org/10.1177/1368430207088035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae K, Misra S, Prasad AK, Pereira SC, Gross JJ. Bottom-up and top-down emotion generation: Implications for emotion regulation. Social, Cognitive & Affective Neuroscience. 2011;7:253–262. doi: 10.1093/scan/nsq103. http://dx.doi.org/10.1093/scan/nsq103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, Akiskal HS, Angst J, Greenberg PE, Hirschfeld RMA, Petukhova M, Kessler RC. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Archives of General Psychiatry. 2007;64:543–552. doi: 10.1001/archpsyc.64.5.543. http://dx.doi.org/10.1001/archpsyc.64.5.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesquita B, Karasawa M. Different emotional lives. Cognition and Emotion. 2002;16:127–141. http://dx.doi.org/10.1080/0269993014000176. [Google Scholar]
- Meyer RE. Conditioning phenomena and the problem of relapse in opioid addicts and alcoholics. In: Ray BA, editor. Learning factors in substance abuse. Washington, DC: U.S. Government Printing Office; 1988. pp. 161–179. [PubMed] [Google Scholar]
- Meyer TD, Baur M. Positive and negative affect in individuals at high and low risk for bipolar disorders. Journal of Individual Differences. 2009;30:169–175. http://dx.doi.org/10.1027/1614-0001.30.3.169. [Google Scholar]
- Meyer B, Johnson SL, Winters R. Responsiveness to threat and incentive in bipolar disorder: Relations of the BIS/BAS scales with symptoms. Journal of Psychopathology and Behavioral Assessment. 2001;23:133–143. doi: 10.1023/A:1010929402770. http://dx.doi.org/10.1023/A:1010929402770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto Y, Ma X. Dampening or savoring positive emotions: A dialectical cultural script guides emotion regulation. Emotion. 2011;11:1346–1357. doi: 10.1037/a0025135. http://dx.doi.org/10.1037/a0025135. [DOI] [PubMed] [Google Scholar]
- Morgan CJ, Badawy AB. Alcohol-induced euphoria: exclusion of serotonin. Alcohol and Alcoholism. 2001;36:22–25. doi: 10.1093/alcalc/36.1.22. http://dx.doi.org/10.1093/alcalc/36.1.22. [DOI] [PubMed] [Google Scholar]
- Munafò MR, Brown SM, Hariri AR. Serotonin transporter (5HTTLPR) genotype and amygdale activation: A meta-analysis. Biological Psychiatry. 2008;63:852–857. doi: 10.1016/j.biopsych.2007.08.016. http://dx.doi.org/10.1016/j.biopsych.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Kitanishi K, Miyake Y, Hashimoto K, Kubota M. The neurotic versus delusional subtupe of taijin-kyofu-sho: Their DSM diagnoses. Psychiatry and Clinical Neurosciences. 2002;56:595–601. doi: 10.1046/j.1440-1819.2002.01061.x. http://dx.doi.org/10.1046/j.1440-1819.2002.01061.x. [DOI] [PubMed] [Google Scholar]
- Nee DE, Wager TD, Jonides J. Interference resolution: Insights from a meta-analysis of neuroimaging tasks. Cognitive Affective & Behavioral Neuroscience. 2007;7:1–17. doi: 10.3758/cabn.7.1.1. http://dx.doi.org/10.3758/CABN.7.1.1. [DOI] [PubMed] [Google Scholar]
- Nesse RM, Berridge KC. Psychoactive drug use in evolutionary perspective. Science. 1997;278:63–66. doi: 10.1126/science.278.5335.63. http://dx.doi.org/10.1126/science.278.5335.63. [DOI] [PubMed] [Google Scholar]
- Novembre J, Di Rienzo A. Spatial patterns of variation due to natural selection in humans. Nature Reviews Genetics. 2009;10:745–755. doi: 10.1038/nrg2632. http://dx.doi.org/10.1038/nrg2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. Cognitive emotion regulation: Insights from social cognitive and affective neuroscience. Current Directions in Psychological Science. 2008;17:153–158. doi: 10.1111/j.1467-8721.2008.00566.x. http://dx.doi.org/10.1111/j.1467-8721.2008.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohman A, Mineka S. Fear, phobias, and preparedness: Toward an evolvedmodule of fear and fear learning. Psychological Review. 2001;108:483–522. doi: 10.1037/0033-295x.108.3.483. http://dx.doi.org/10.1037/0033-295X.108.3.483. [DOI] [PubMed] [Google Scholar]
- Okazaki S, Liu JF, Longworth SL, Minn JM. Asian American-White American differences in expressions of social anxiety: A replication and extension. Cultural Diversity and Ethnic Minority Psychology. 2002;8:234–247. doi: 10.1037/1099-9809.8.3.234. http://dx.doi.org/10.1037/1099-9809.8.3.234. [DOI] [PubMed] [Google Scholar]
- Oveis C, Cohen AB, Gruber J, Shiota MN, Haidt J, Keltner D. Resting respiratory sinus arrhythmia is associated with tonic positive emotionality. Emotion. 2009;9:265–270. doi: 10.1037/a0015383. http://dx.doi.org/10.1037/a0015383. [DOI] [PubMed] [Google Scholar]
- Park DC, Gutchess AH. Aging, cognition, and culture: A neuroscientific perspective. Neuroscience and Biobehavioral Reviews. 2002;26:859–867. doi: 10.1016/s0149-7634(02)00072-6. http://dx.doi.org/10.1016/S0149-7634(02)00072-6. [DOI] [PubMed] [Google Scholar]
- Park DC, Gutchess AH. The cognitive neuroscience of aging and culture. Current Directions in Psychological Science. 2006;15:105–108. http://dx.doi.org/10.1111/j.0963-7214.2006.00416.x. [Google Scholar]
- Peeters F, Nicholson NA, Berkhof J. Cortisol responses to daily events in major depressive disorder. Psychosomatic Medicine. 2003;65:836–841. doi: 10.1097/01.psy.0000088594.17747.2e. http://dx.doi.org/10.1097/01.PSY.0000088594.17747.2E. [DOI] [PubMed] [Google Scholar]
- Pew Research Center Social and Demographic Trends. U.S. population projections: 2005–2050. 2008 Available at: http://pewsocialtrends.org/pubs/703/population-projections-united-states.
- Phillips ML, Ladouceur CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: Implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mollecular Psychiatry. 2008;13:829–857. doi: 10.1038/mp.2008.65. http://dx.doi.org/10.1038/mp.2008.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW. Vagal tone: A physiological marker of stress vulnerability. Pediatrics. 1992;90:498–504. [PubMed] [Google Scholar]
- Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, Goodwin FK. Comorbidity of mental disorders with alcohol and other drug abuse: Results from the epidemiologic catchment area (ECA) study. Journal of the American Medical Association. 1990;264:2511–2518. http://dx.doi.org/10.1001/jama.1990.03450190043026. [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory ofaddiction. Brain Research Reviews. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. http://dx.doi.org/10.1016/0165-0173(93)90013-P. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction. Annual Review of Psychology. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. http://dx.doi.org/10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The incentive sensitization theory of addiction: Some current issues. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2008;363:3137–3146. doi: 10.1098/rstb.2008.0093. http://dx.doi.org/10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottenberg J, Gross JJ, Gotlib IH. Emotion context insensitivity in major depressive disorder. Journal of Abnormal Psychology. 2005;114:627–639. doi: 10.1037/0021-843X.114.4.627. http://dx.doi.org/10.1037/0021-843X.114.4.627. [DOI] [PubMed] [Google Scholar]
- Rottenberg J, Kasch KL, Gross JJ, Gotlib IH. Sadness and amusement reactivity differentially predict concurrent and prospective functioning in major depressive disorder. Emotion. 2002;2:135–146. doi: 10.1037/1528-3542.2.2.135. http://dx.doi.org/10.1037/1528-3542.2.2.135. [DOI] [PubMed] [Google Scholar]
- Rosenberg NA, Huang L, Jewett EM, Szpiech ZA, Jankovic I, Boehnke M. Genome-wide association studies in diverse populations. Nature Reviews Genetics. 2010;11:356–366. doi: 10.1038/nrg2760. http://dx.doi.org/10.1038/nrg2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxbe DE, Yang XF, Borofsky LA, Immordino-Yang MH. The embodiment of emotion: Language use during the feeling of social emotions predicts cortical somatosensory activity. Social Cognitive and Affective Neuroscience. 2012 doi: 10.1093/scan/nss075. Advance online publication. http://dx.doi.org/10.1093/scan/nss075. [DOI] [PMC free article] [PubMed]
- Schneider U, Altmann A, Baumann M, Bernzen B, Bertz B, Bimber U, Wittfoot J. Comorbid anxiety and affective disorder in alcohol-dependent patients seeking treatment: the first multicentre study in Germany. Alcohol and Alcoholism. 2001;36:219–223. doi: 10.1093/alcalc/36.3.219. http://dx.doi.org/10.1093/alcalc/36.3.219. [DOI] [PubMed] [Google Scholar]
- Schreckenberger M, Amberg R, Scheurich A, Lochmann M, Tichy W, Kelga A, Urban R. Acute alcohol effects on neuronal and attentional processing: Striatal reward system and inhibitory sensory interactions under acute ethanol challenge. Neuropsychopharmacology. 2004;29:1527–1537. doi: 10.1038/sj.npp.1300453. http://dx.doi.org/10.1038/sj.npp.1300453. [DOI] [PubMed] [Google Scholar]
- Silvers JA, McRae K, Gabrieli JDE, Gross JJ, Remy KA, Ochsner KN. Age-related differences in emotional reactivity, regulation, and rejection sensitivity in adolescence. Emotion. 2012 doi: 10.1037/a0028297. Advance online publication. http://dx.doi.org/10.1037/a0028297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singelis TM. The measurement of independent and interdependent self-construals. Personality and Social Psychology Bulletin. 1994;20:50–591. http://dx.doi.org/10.1177/0146167294205014. [Google Scholar]
- Sloan DM, Strauss ME, Wisner KL. Diminished response to pleasant stimuli by depressed women. Journal of Abnormal Psychology. 2001;110:488–493. doi: 10.1037//0021-843x.110.3.488. http://dx.doi.org/10.1037/0021-843X.110.3.488. [DOI] [PubMed] [Google Scholar]
- Smelser N, Wilson WJ, Mitchell F. Introduction. In: Smelser N, Wilson W, Mitchell F, editors. America becoming: Racial trends and their consequences. Washington, DC: National Academy Press; 2001. pp. 1–20. [Google Scholar]
- Spanagel R, Weiss F. The dopamine hypothesis of reward: past and current status. Trends in Neuroscience. 1999;22:521–7. doi: 10.1016/s0166-2236(99)01447-2. http://dx.doi.org/10.1016/S0166-2236(99)01447-2. [DOI] [PubMed] [Google Scholar]
- Swendsen JD, Merikangas KR, Caino GJ, Kessler RC, Rubio-Stipec M, Angst J. The comorbidity of alcoholism with anxiety and depressive disorders in four geographic communities. Comprehensive Psychiatry. 1998;39:176–184. doi: 10.1016/s0010-440x(98)90058-x. http://dx.doi.org/10.1016/S0010-440X(98)90058-X. [DOI] [PubMed] [Google Scholar]
- Takeuchi DT, Chung RC, Lin K, Shen H, Kurasaki K, Chun C, Sue S. Lifetime and twelvemonth prevalence rates of major depressive episodes and dysthymia among Chinese Americans in Los Angeles. American Journal of Psychiatry. 1998;155:1407–1414. doi: 10.1176/ajp.155.10.1407. [DOI] [PubMed] [Google Scholar]
- Tamir M, John OP, Srivastava S, Gross JJ. Implicit theories of emotion: Affective and social outcomes across a major life transition. Journal of Personality and Social Psychology. 2007;92:731–744. doi: 10.1037/0022-3514.92.4.731. http://dx.doi.org/10.1037/0022-3514.92.4.731. [DOI] [PubMed] [Google Scholar]
- Tangney JP, Tracy JL. Self-conscious emotions. In: Leary M, Tangney JP, editors. Handbook of self and identity. 2nd ed. New York, NY: Guilford; 2012. pp. 446–478. [Google Scholar]
- Tiffany ST. A cognitive model of drug urges and drug-use behavior: Role of automatic and nonautomatic processes. Psychological Review. 1990;97:147–168. doi: 10.1037/0033-295x.97.2.147. http://dx.doi.org/10.1037/0033-295X.97.2.147. [DOI] [PubMed] [Google Scholar]
- Tracy JL, Matsumoto D. The spontaneous expression of pride and shame: Evidence for biologically innate nonverbal displays. Proceedings of the National Academy of Sciences. 2008;105:11655–11660. doi: 10.1073/pnas.0802686105. http://dx.doi.org/10.1073/pnas.0802686105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy JL, Robins RW. The nonverbal expression of pride: Evidence for cross-cultural recognition. Journal of Personality and Social Psychology. 2008;94:516–530. doi: 10.1037/0022-3514.94.3.516. http://dx.doi.org/10.1037/0022-3514.94.3.516. [DOI] [PubMed] [Google Scholar]
- Tsai JL. Ideal affect: Cultural causes and behavioral consequences. Perspectives on Psychological Science. 2007;2:242–259. doi: 10.1111/j.1745-6916.2007.00043.x. http://dx.doi.org/10.1111/j.1745-6916.2007.00043.x. [DOI] [PubMed] [Google Scholar]
- Tsai JL, Knutson B, Fung HH. Cultural variation in affect valuation. Journal of Personality and Social Psychology. 2006;90:288–307. doi: 10.1037/0022-3514.90.2.288. http://dx.doi.org/10.1037/0022-3514.90.2.288. [DOI] [PubMed] [Google Scholar]
- Tugade MM, Fredrickson BL. Resilient individuals use positive emotions to bounce back from negative emotional experiences. Journal of Personality and Social Psychology. 2004;86:320–333. doi: 10.1037/0022-3514.86.2.320. http://dx.doi.org/10.1037/0022-3514.86.2.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk CL, Heimberg RG, Luterek JA, Mennin DS, Fresco DM. Emotion dysregulation in generalized anxiety disorder: A comparison with social anxiety disorder. Cognitive Therapy and Research. 2005;29:89–106. http://dx.doi.org/10.1007/s10608-005-1651-1. [Google Scholar]
- Wager TD, Reading S, Jonides J. Neuroimaging studies of shifting attention: a meta-analysis. Neuroimage. 2004;22:1679–1693. doi: 10.1016/j.neuroimage.2004.03.052. http://dx.doi.org/10.1016/j.neuroimage.2004.03.052. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Carey G. Positive and negative affectivity and their relation to anxiety and depressive disorders. Journal of Abnormal Psychology. 1988;97:346–353. doi: 10.1037//0021-843x.97.3.346. http://dx.doi.org/10.1037/0021-843X.97.3.346. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. http://dx.doi.org/10.1037/0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Wearden AJ, Tarrier N, Barrowclough C, Zastowny TR, Rahill AA. A review of expressed emotion research in health care. Clinical Psychology Review. 2000;20:633–666. doi: 10.1016/s0272-7358(99)00008-2. http://dx.doi.org/10.1016/S0272-7358(99)00008-2. [DOI] [PubMed] [Google Scholar]
- Weber EU, Hsee CK. Culture and individual judgment and decision-making. Journal of Applied Psychology. 2000;49:32–61. http://dx.doi.org/10.1111/1464-0597.00005. [Google Scholar]
- Weissman MM, Bland RC, Canino GJ, Faravelli C, Greenwald S, Hwu H, Joyce PR, Yeh E. Cross-national epidemiology of major depression and bipolar disorder. Journal of the American Medical Association. 1996;276:293–299. http://dx.doi.org/10.1001/jama.1996.03540040037030. [PubMed] [Google Scholar]
- Wills TA, Shiffman S. Coping and substance use: A conceptual framework. In: Shiffman S, Wills TA, editors. Coping and substance use. New York: Academic Press; 1985. pp. 3–24. [Google Scholar]
- World Health Organization. Global status report on alcohol and health. Geneva, Switzerland: WHO Press; 2011. [Google Scholar]
- The WHO World Mental Health Survey Consortium. Prevalence, severity, and unmet need for treatment of mental disorders in the World Health Organization World Mental Health Surveys. Journal of the American Medical Association. 2004;291:2581–2590. doi: 10.1001/jama.291.21.2581. http://dx.doi.org/10.1001/jama.291.21.2581. [DOI] [PubMed] [Google Scholar]
- Wrase J, Schlagenhauf F, Kienast T, Wustenberg T, Bermpohl F, Kahnt T, Heinz A. Dysfunction of reward processing correlates with alcohol craving in detoxified alcoholics. NeuroImage. 2007;35:787–794. doi: 10.1016/j.neuroimage.2006.11.043. http://dx.doi.org/10.1016/j.neuroimage.2006.11.043. [DOI] [PubMed] [Google Scholar]
- Yurgelun-Todd DA, Gruber SA, Kanayama G, Killgore WDS, Baird AA, Young AD. fMRI during affect discrimination in bipolar affective disorder. Bipolar Disorders. 2000;2:237–248. doi: 10.1034/j.1399-5618.2000.20304.x. http://dx.doi.org/10.1034/j.1399-5618.2000.20304.x. [DOI] [PubMed] [Google Scholar]
- Wearden AJ, Tarrier N, Barrowclough C, Zastowny TR, Rahill AA. A review of expressed emotion research in health care. Clinical Psychology Review. 2000;20:633–666. doi: 10.1016/s0272-7358(99)00008-2. http://dx.doi.org/10.1016/S0272-7358(99)00008-2. [DOI] [PubMed] [Google Scholar]
- Zhou H, Cacioppo J. Culture and the brain: Opportunities and obstacles. Asian Journal of Social Psychology. 2010;13:59–71. [Google Scholar]