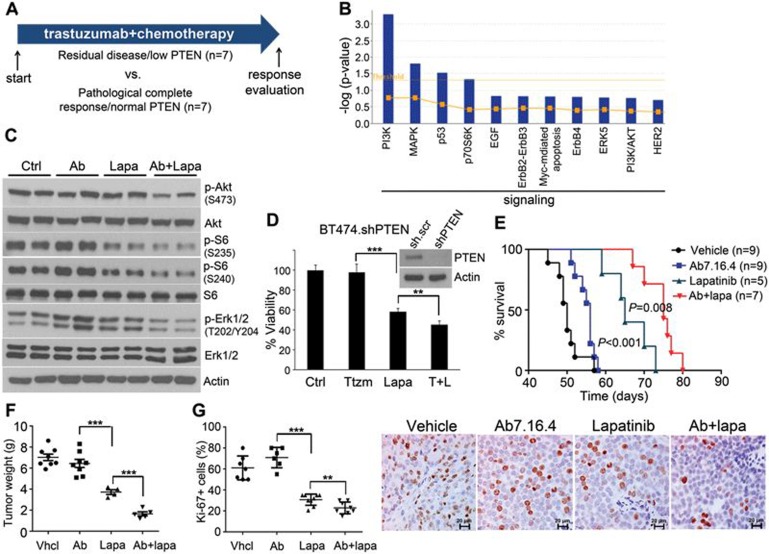
Figure 1.
Lapatinib alone or combined with anti-ErbB2 antibody Ab7.16.4 increases survival of PTEN−/−/NIC trastuzumab-resistant mice. (A) Treatment scheme of 14 ErbB2-positive breast cancer patients. Patients were classified into two groups: trastuzumab sensitive with high PTEN level (PCR/high PTEN) (n = 7) and trastuzumab resistant with normal PTEN level (RD/low PTEN) (n = 7). (B) Identification of significantly different canonical pathways between trastuzumab-resistant and -sensitive patients using IPA. Threshold is at P < 0.05 (dotted yellow line); y axis is −log of the P-value. (C) Western blot of PTEN−/−/NIC tumor lysates from mice treated with different drugs for 3 days. (D) WST-1 cell proliferation assay comparing proliferation of BT474.shPTEN cells after drug treatment for 72 h (Ttzm: trastuzumab and T + L: trastuzumab plus lapatinib). Inset shows western blot validation of PTEN knockdown. **P < 0.01, ***P < 0.001 by two-tailed t-test. (E) Survival analysis of the PTEN−/−/NIC mice treated with different drugs or combinations. P < 0.001 (by log-rank test) is between Ab7.16.4 and lapatinib; P = 0.008 is between lapatinib and Ab7.16.4 + lapatinib. (F) Left, tumor weight (mean ± SEM) of mice treated with vehicle, 7.16.4 mAb (Ab), lapatinib, or combination for 3 weeks. ***P < 0.001 by two-tailed t-test. (G) Ki-67 staining of tumor tissue obtained from mice treated with different drugs or their combination. Scale bar indicates 20 μm. **P < 0.01, ***P < 0.001 by two-tailed t-test.