Table 1. Scope of Michael Addition of 1,1,1-Trifluoromethylketones 1 to Nitroolefins 2a.
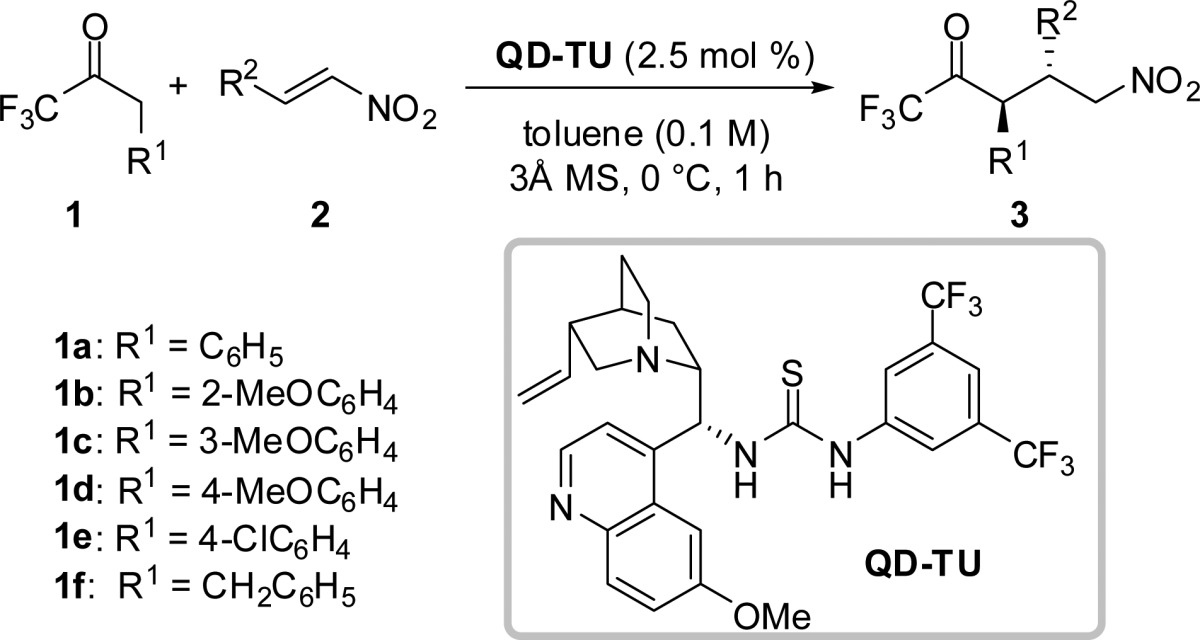
| entry | 1 | 2 | 3 | yield (%)b | drc | erd |
|---|---|---|---|---|---|---|
| 1 | 1a | C6H5 | 3aa | 98 | >20:1 | 95:5 |
| 2 | 1a | 2-BrC6H4 | 3ab | 95 | >20:1 | 96.5:3.5 |
| 3 | 1a | 2-NO2C6H4 | 3ac | 94 | >20:1 | 97.5:2.5 |
| 4 | 1a | 2-CF3C6H4 | 3ad | 96 | >20:1 | 96.5:3.5e |
| 5 | 1a | 2-OMeC6H4 | 3ae | 98 | >20:1 | 94:6 |
| 6 | 1a | 3-ClC6H4 | 3af | 98 | 19:1 | 93.5:6.5 |
| 7 | 1a | 4-BrC6H4 | 3ag | 99 | >20:1 | 95.5:4.5 |
| 8 | 1a | 4-NO2C6H4 | 3ah | 99 | 7:1 | 95.5:4.5 |
| 9 | 1a | 4-CNC6H4 | 3ai | 96 | >20:1 | 96:4 |
| 10 | 1a | 4-MeC6H4 | 3aj | 96 | 8:1 | 93:7 |
| 11 | 1a | 4-OMeC6H4 | 3ak | 91 | 6:1 | 86.5:13.5 |
| 12 | 1a | 2-thienyl | 3al | 97 | 16:1 | 91.5:8.5 |
| 13f | 1a | 3-N-Ts-indoyl | 3am | 92 | >20:1 | 87.5:12.5 |
| 14 | 1a | CH=CHC6H5 | 3an | 62 (65)g | >20:1 | 87:13 |
| 15 | 1a | cyclohexyl | 3ao | 42 (43)g | >20:1 | 94:6 |
| 16 | 1b | 4-BrC6H4 | 3bg | 97 | >20:1 | 96:4 |
| 17 | 1c | 4-BrC6H4 | 3cg | 96 | >20:1 | 88:12 |
| 18 | 1d | 4-BrC6H4 | 3dg | 97 | >20:1 | 87:13 |
| 19 | 1e | 4-BrC6H4 | 3eg | 95 | >20:1 | 93:7 |
| 20h | 1f | C6H5 | 3fa | 73 (82)g | >20:1 | 97:3 |
Reactions were performed with 1 (0.21 mmol) and 2 (0.20 mmol) and proceeded to full conversion as adjudged by TLC.
Isolated yield. The diastereomers were not separable, and this represents the combined yield.
The diastereomeric ratio was determined by 19F NMR spectroscopic analysis of the crude product.
The enantiomeric ratio was determined by HPLC or SFC analysis on a chiral stationary phase.
The enantiomeric ratio was determined following reduction of 3ad with NaBH4 (see the SI).
The reaction was performed at 0 °C for 3 h.
Number in parentheses is conversion of nitroolefin as determined by 1H NMR spectroscopic analysis of the crude product.
The reaction was performed employing QD-TU (10 mol %) at 0 °C for 12 h.
