Abstract
Introduction
Priapism is a poorly understood disease process with little information on the etiology and pathophysiology of this erectile disorder. One group of patients with a high prevalence of priapism is men with sickle-cell disease.
Aim
Establish an in vivo transgenic sickle-cell mouse model to study the pathophysiology of sickle-cell disease-associated priapism.
Methods
Transgenic sickle-cell disease mice, expressing human sickle hemoglobin, were utilized. Three groups of mice were used: (i) wild type (WT), (ii) sickle-cell heterozygotes (Hemi), and (ii) sickle-cell homozygotes (Sickle). Two age groups of each cohort of mice were utilized: young adult (4–6 months) and aged (18–22 months).
Main Outcome Measures
Histological (trichrome stain to measure ratio of collagen to smooth muscle), penile hydroxyproline content (collagen content), and transmission electron microscopic analysis of WT, Hemi, and Sickle mice penes, as well as in vivo erectile responses [change in intracavernous pressure (ICP)] to cavernous nerve stimulation (CNS), were determined. The frequency of erectile responses (erections/hour) pre- and poststimulation was also measured in each of the experimental groups.
Results
Sickle mice had increased (P < 0.05) collagen to smooth muscle ratio and hydroxyproline content in the penis when compared with WT and Hemi mice penes. Transmission electron microscopy demonstrated thickened smooth muscle cell bundles, disruption of the endothelial lining of the corporal sinusoids, and increased (P < 0.05) caveolae number. Sickle mice had significantly (P < 0.05) higher ICP to CNS and increased (P < 0.05) frequency of erections pre- and post-CNS when compared with WT and Hemi mice erectile responses. Sickle mice did develop ED (change in ICP in response to CNS) with increasing age.
Conclusion
The morphometric changes of the penis and exaggerated in vivo erectile responses support the use of this transgenic sickle-cell disease animal model to study the pathophysiological mechanisms involved in sickle-cell disease-associated priapism.
Keywords: Erectile Dysfunction, Ischemic Priapism, Endothelium, Fibrosis, Cavolae, Nitric Oxide
Introduction
Priapism is defined as an erectile disorder, in which erection persists uncontrollably without sexual purpose [1]. The mechanisms involved in development of priapism are poorly characterized; therefore, medical management of priapism represents a therapeutic challenge to urologists. In the penis of men with ischemic priapism, the corporal bodies and vascular endothelium are exposed to intermittent episodes of anoxia. Thus, cellular, molecular and morphologic changes are activated, which often become maladaptive and contribute to progressive erectile dysfunction (ED). Conventional treatments are largely reactive, usually administered after a single episode of priapism has already occurred. Potential therapeutic targets aimed at preventing recurrent ischemic priapism have been proposed; however, they lack a mechanism-based approach and thus are largely ineffective [1–3]. To date, the only animal models to study the pathophysiology of priapism utilize direct injection of erectogenic agents and knockout mice that manifest priapism phenotypes [4–9]. These early studies have provided great understanding of the pathophysiology of priapism; however, limitations to these initial reports are related to the lack of utilization of animal models that fit with the relevant pathogenic associations of priapism, such as sickle-cell disease.
One category of patients with a high prevalence of recurrent priapism is individuals with sickle-cell disease. The lifetime probability for the development of clinically significant priapism is as high as 42% in men with sickle-cell disease, and the rate of resultant ED exceeds 30% [10–12]. The identification of genetic variables or molecular mechanisms that precondition the sickle-cell penile vasculature to respond abnormally to any degree of sexual stimulus is unclear. Early reports have suggested that transgenic sickle-cell mice have episodes of priapism; however, the measurement of erectile function was not critically evaluated, and thus the conclusion is not valid [13]. Recently, Mi et al. have shown that transgenic sickle-cell mice corpora cavernosa have enhanced smooth muscle relaxation to electrical field stimulation, which supports that sickle-cell mice may have a priapism phenotype in vivo [9]. The present study was designed to characterize the morphological changes that occur in the penis and the in vivo erectile responses to neurogenic stimulation in a transgenic sickle-cell disease mouse model. The establishment of an in vivo animal model and vigorous preclinical analyses are imperative in order to ultimately study the pathophysiology of sickle disease-associated ischemic priapism.
Material and Methods
Mouse Model of Human Sickle-Cell Disease
Transgenic sickle-cell (Sickle) mice with knockout of all mouse hemoglobin genes and expressing exclusively human sickle hemoglobin were developed at Lawrence Berkeley National Laboratory [14,15]. A breeding colony at the National Institutes of Health (NIH) generated animals for this study by mating sickle-male mice to hemizygous females (approximately 15 generations). Because C57BL/6 is one of the background strains for the transgenic sickle mice, C57BL/6 was chosen as wild-type (WT) control. Additional control animals were hemizygous (Hemi) littermates, which have anemia and increased oxidative stress but no sickle deformation [14]. All were males ages 4 to 6 months old, except a series of retired breeder males (18 to 22 months old) to determine the effects of aging. Mice were pathogen free and received routine NIH rodent chow and water. Studies were approved by the animal care and use committees of Johns Hopkins Medical Institutions.
Histology and Electron Microscopy
WT, Hemi, and Sickle penes (4–6 months) were fixed with 10% formalin overnight, then embedded in paraffin, sectioned at a thickness of 5 μm and stained with Masson’s trichrome stain as previously described [16]. For measurement of collagen to smooth muscle ratio (fibrosis scale), an independent pathologist blinded as to tissue group, graded on a scale of 1 (least) to 5 (severe) the amount of increase in collagen to smooth muscle ratio. For transmission electron microscopy, penes were immersion fixed in 2% glutaraldehyde and 0.2 M sodium phosphate buffer, pH 7.4, overnight. After fixation, the samples were cyroprotected and prepared for transmission electron microscopic analysis as previously described [17]. For quantitation of caveolae, 12–15 cells were photographed at a magnification of 40,000×. The average number of caveolae per μm of sectioned plasma membrane is expressed as mean ± standard error of the mean (SEM) [18]. Caveolae were counted by three independent blinded observers who used the original photographs.
Penile Hydroxyproline Content
Penile collagen content was quantitated by measuring the total hydroxyproline content of the penis. Penile hydroxyproline concentration was determined spectrophotometrically as previously described [19]. Briefly, WT, Hemi, and Sickle penes were homogenized in 5% trichloroacetic acid (1:9, wt/vol), and centrifuged for 10 minutes at 4000 g. The pellet was then washed twice with distilled water and hydrolyzed for 16 hour at 100°C in 6 N HCl. Hydroxyproline in the hydrolysate was assessed colorimetrically at 561 nm with p-dimethylaminobenzaldehyde. Hydroxyproline content was computed as micrograms of hydroxyproline per penis and indexed to body weight [19].
Physiologic Erection Studies
In vivo erectile function in response to cavernous nerve stimulation (CNS) was studied in WT, Hemi, and Sickle anesthetized mice. Induction of anesthesia was achieved by placing the animal in a jar containing gauze soaked with isoflurane. The mice were then intubated and placed on a thermoregulated surgical table. The animals were ventilated with 95% O2/5% CO2 and 2% isoflurane using a custom-designed, constant-flow mouse ventilator with tidal volume set to 6.7 μL/g at 140 breaths/min. A carotid artery was cannulated for measurement of mean systemic arterial pressure (MAP), which was measured continuously with a Viggo-Spectramed transducer (Viggo Spectramed, Oxnard, CA, USA) attached to a data acquisition system (Biopac Systems, Santa Barbara, CA, USA). Heart rate was determined from the systolic pressure pulses with a tachometer (Biopac). MAP was measured in only a small cohort of mice from each group.
The shaft of the penis was freed of skin and fascia, and by removing part of the overlying ischiocavernous muscle, exposure of the right crus was performed. A 27-gauge needle filled with 250 U/mL of heparin and connected to PE-50 tubing was inserted into the right crura and connected to a pressure transducer to permit continuous measurement of intracavernosal pressure (ICP). The bladder and prostate were exposed through a midline abdominal incision. The right major pelvic ganglion and cavernous nerve were identified posterolateral to the prostate on one side, and an electrical stimulator with a stainless steel bipolar hook was placed around the cavernous nerve. ICP was measured with a pressure transducer connected to a data acquisition system (Biopac) for continuous measurement of ICP. The cavernous nerve was stimulated with a square pulse stimulator (Grass Instruments, Quincy, MA, USA). Each mouse underwent CNS at a frequency of 15 Hz and pulse width of 30 ms. The application of 1, 2, and 4 V was used in the current protocol to achieve a significant and consistent erectile response. The duration of stimulation was approximately 30 seconds, with rest periods of 2–3 minutes between subsequent stimulations. This procedure has been previously described [20]. In Sickle cell mice experiencing prolonged erections with increases in ICP > 33% of peak ICP pressure to maximal CNS (2 V), the frequency of erections per hour was calculated pre and post-CNS (2 V).
Statistical Analysis
Data are presented as mean ± SEM. Comparisons between baseline variables in WT, Hemi, and Sickle mice were performed using paired or unpaired t-tests, as appropriate. Comparisons between groups were made using anova analysis with repeated measures and Neumann–Keuls post hoc test for multiple group comparisons. Statistical calculations were performed using GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, CA, USA, http://www.graphpad.com).
Results
Morphometric, Hydroxyproline, and Electron Microscopic Analysis
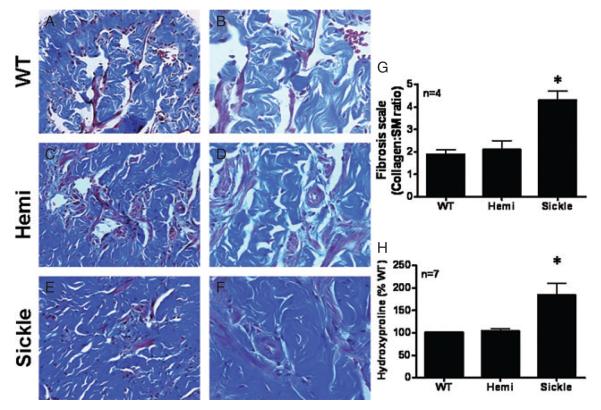
The final sequelae of chronic anoxia and ischemia of priapism is resultant severe corporal smooth muscle and endothelial damage and widespread necrosis [21]. In the first series of experiments, WT, Hemi, and Sickle mice penes were subjected to histological and electron microscopic analysis to determine if Sickle mice penes had end-organ changes consistent with the morphometric changes that occur in penes of men with ischemic priapism. Adult male (4 to 6 months) Sickle mice penes (Figure 1E, F) had an increase in collagen to smooth muscle ratio (Figure 1G) when compared with WT (Figure 1A, B) and sickle-cell heterozygotes (Hemi; Figure 1C, D) as measured by Masson’s trichrome stain. Additionally, the amount of hydroxyproline content (collagen content) was significantly more (P < 0.05) in Sickle mice penes (Figure 1) when compared with WT and Hemi mice penes. These data suggest that Sickle mice penes have increased hydroxyproline content and ratio of collagen to smooth muscle (fibrosis) of the penis (Figure 1).
Figure 1.
Masson’s trichrome stain wild type (WT) (A, B), heterozygous (Hemi; C, D) and transgenic sickle-cell (Sickle; E, F) mice penes. Magnification 20× (A, C, E) and 40× (B, D, F). (G) Bar graph demonstrating the ratio of collagen to smooth muscle (SM) in all experimental groups. (H) Bar graph demonstrating quantitative assessment of cavernosal collagen by hydroxyproline assay in WT, Hemi, and Sickle mice. n = number of experiments; *P < 0.05 vs. WT and Hemi. Data expressed as mean values ± SEM. SEM = standard error of the mean.
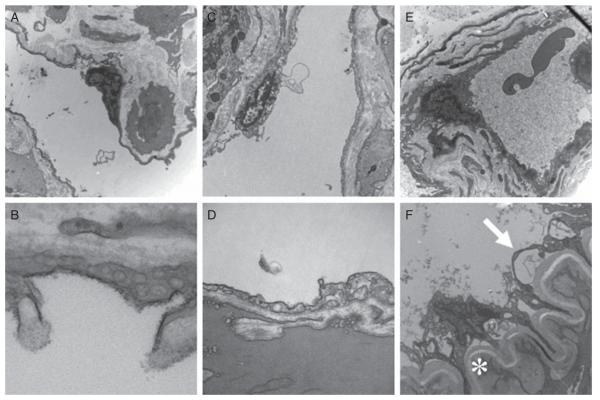
In the corpora cavernosa, numerous endothelial cells line the corporal sinusoids and blood vessels (arterial and venous). In WT and Hemi mice penes, cavernous sinusoids and capillaries were lined with intact endothelium (Figure 2A–D). In Sickle mice penes, thickened smooth muscle cell bundles with thickened basement membranes (star) and disruption of the endothelial lining (arrow) of the corporal sinusoids were observed (Figure 2E, F) when compared with WT (Figure 2A, B) and Hemi mice penes (Figure 2C, D), paralleling the morphometric findings. There was sickle hemoglobin in the corporal sinusoids of Sickle mice penes (Figure 2E) and increased collagen deposition (Figure 2E). In Sickle mice corporal sinusoids, there was evidence of endothelial breakdown with increased adherence of red blood cells (RBC) to the lining of the endothelium lining the corporal sinusoids (Figure 2F).
Figure 2.
Electron microscopic analysis of wild type (WT) (A, B), heterozygous (Hemi; C, D) and transgenic sickle-cell (Sickle; E, F) mice penes. Magnification 8,000× (A, C, E) and 40,000× (B, D, F); asterisk (*) in 2F represents smooth muscle cell with increased collagen deposition and thickening of basement membrane; arrow represents endothelial lining. Images are representative of three to four penile samples.
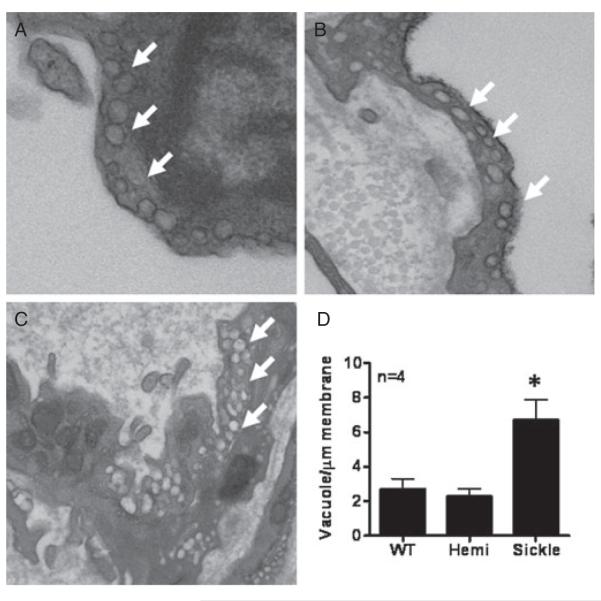
To further evaluate the integrity of the corporal endothelium in WT, Hemi, and Sickle mice penes, we qualitatively studied prominent changes in ultrastructure by electron microscopy (Figure 3). The caveolae appeared as noncoated plasma-lemmal vesicles with a round lumen of up to 50–100 nm in diameter (Figure 3). Caveolae were prevalent in WT and Hemi mice penile sinusoids in both the luminal and abluminal sides of the cells (Figure 3A, B). However, Sickle mice penes displayed increased numbers of vacuoles with a less vesicle-shaped appearance (Figure 3C). To determine the significance of these changes, caveolae number in WT and Hemi mice penes or vacuoles in Sickle mice was quantified by counting the numbers of vacuole per μm membrane length. Quantitation revealed significant increases (P < 0.05) in vacuole number in Sickle cell mice penes (6.7 ± 1.2) when compared with WT (2.7 ± 0.6) and Hemi mice (2.3 ± 0.4) penile samples (Figure 3D). These findings suggest that formation and stability of caveolae are altered by sickle-cell disease.
Figure 3.
Ultrastructure of the endothelium using transmission electron microscopy in wild type (WT) (A), heterozygous (Hemi; B), and transgenic sickle-cell (Sickle; C) mice penes. Magnification 40,000×. Arrows denote vacuoles. (D) Quantitative assessment of vacuole density per μg membrane length. Measurements were made in blinded fashion. Images are representative of four penile samples. n indicates number of experiments. *P < 0.05 vs. WT and Hemi.
In Vivo Erectile Responses
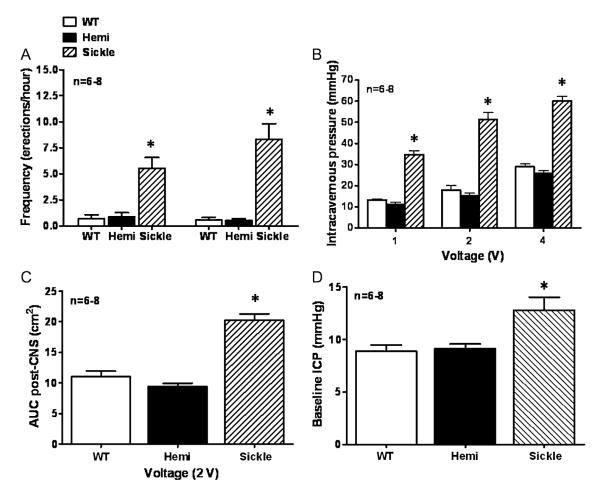
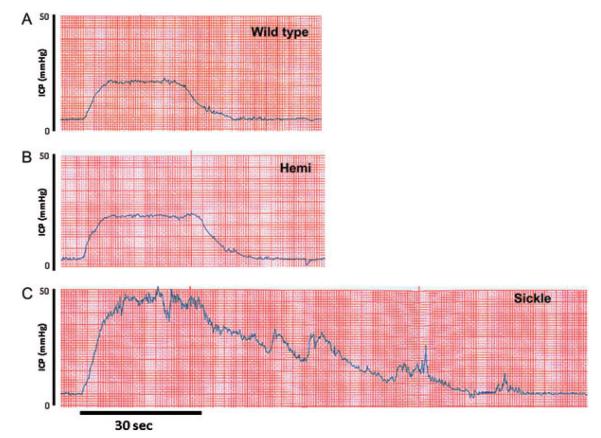
In the next series of experiments, erectile responses to CNS were conducted to evaluate in vivo erectile physiology and determine if the Sickle mice displayed a priapic phenotype (Figure 4). Sickle mice had elevated resting ICP (P < 0.05) when compared with WT and Hemi mice, suggesting increased corporeal perfusion at baseline (Figure 4D). Sickle mice displayed priapic activity and pronounced erectile responses to CNS at all voltage settings, as well as variably phasic and prolonged erections after discontinuation of neuro-stimulation (P < 0.05; Figure 4A, B). Sickle mice had more episodes of priapic activity (erections/hour) defined as the spontaneous increase in ICP pre- and post-CNS (2 V) when compared with WT and Hemi mice (P < 0.05; Figure 4A). In both WT and Hemi mice, ICP increased incrementally over a standard voltage range, with a maximal response at 4 V. However, Sickle mice were highly responsive to CNS even at low voltage, achieving maximal penile erection with a 2-V stimulus (Figures 4B, 5C). The detumescence phase of the erectile response after CNS termination (2 V) was also significantly increased in Sickle mice (P < 0.05; Figure 4C). Figure 5 demonstrates representative in vivo changes in ICP to CNS (2 V) in WT, Hemi, and Sickle mice. Sickle mice have enhanced peak ICP response to CNS (2 V), as well as prolonged detumescence phase of the erectile response and spontaneous increases in ICP (Figure 5). These data establish in vivo evidence of priapism in Sickle mice. Resting MAP and heart rate were similar between all young age-matched WT, Hemi, and Sickle mice (Table 1). There was a significant increase (P < 0.05) in MAP in the aged WT, Hemi, and Sickle mice when compared with the young cohort of mice (Table 1). Additionally, aged Sickle mice had significantly higher (P < 0.05) MAP when compared with aged WT and Hemi mice (Table 1).
Figure 4.
(A) Bar graph demonstrating the frequency of erections (erections/hour) pre- and postcavernous nerve stimulation (CNS; 2 V) in wild type (WT), heterozygous (Hemi), and transgenic sickle-cell (Sickle) mice; (B) Voltage dependent erectile responses to CNS; (C) area under the erectile curve (cm2) post-CNS (2 V); (D) baseline resting intracavernous pressure. *P < 0.05 vs. WT and Hemi. For all panels, data expressed as mean values ± SEM. n = number of experiments; SEM = standard error of the mean.
Figure 5.
Representative intracavernous pressure (ICP) tracing in response to cavernous nerve stimulation (2 V for 30 seconds) in young wild type (WT) (A), heterozygous (B; Hemi), and transgenic sickle-cell (C; Sickle) mice.
Table 1.
Mean systemic arterial pressure (MAP) and heart rate (HR) in young (n = 5) and aged (n = 5–6) wild type (WT), heterozygotes (Hemi), and transgenic sickle-cell (Sickle) mice
| MAP (mm Hg) | HR (beats/min) | |
|---|---|---|
| Young WT | 83.8 ± 4.7 | 585.8 ± 12.7 |
| Young Hemi | 85.8 ± 2.6 | 579.5 ± 10.2 |
| Young Sickle | 81.5 ± 5.3 | 582.8 ± 9.0 |
| Aged WT | 95.7 ± 5.5* | 584.4 ± 21.1 |
| Aged Hemi | 96.7 ± 5.5** | 587.3 ± 13.9 |
| Aged Sickle | 106.8 ± 2.7***;**** | 592.5 ± 8.3 |
P = 0.0061 young vs. aged WT MAP;
P = 0.0035 young vs. aged Hemi MAP;
P ≤ 0.0001 young vs. aged Sickle MAP;
P = 0.0049 aged sickle vs. aged WT and Hemi MAP.
Effect of Age on In Vivo Erectile Responses in Sickle Mice
In order to study the effect of the natural aging process on priapic activity in Sickle mice, we evaluated the in vivo change in ICP in response to CNS (2 V) in young (4–6 months) and aged (18–22 months) WT, Hemi, and Sickle mice (Figure 6). Young Sickle mice had significantly exaggerated (P < 0.05) erectile responses (changes in ICP) in response to CNS when compared with WT and Hemi mice (Figure 6). Aged WT, Hemi, and Sickle mice displayed significantly reduced (P < 0.05) erectile responses to CNS when compared with young WT, Hemi, and Sickle mice, respectively (Figure 6). Aged Sickle mice had significantly reduced in vivo erectile responses to CNS when compared with young WT and Hemi mice (Figure 6). These data suggest that as Sickle mice age, they lose their priapism phenotype and develop ED, which is consistent with the natural course of disease in patients with sickle-cell disease-associated priapism.
Figure 6.
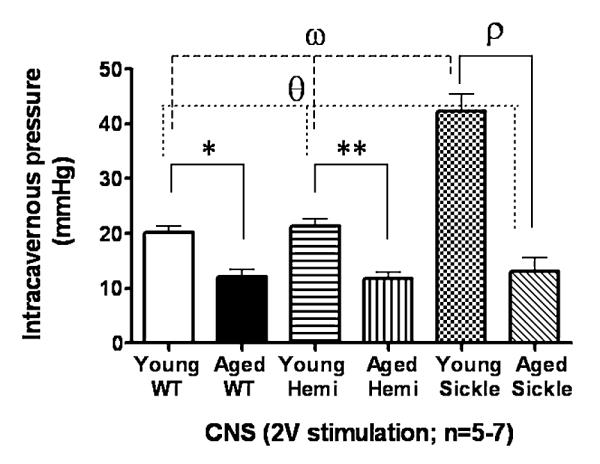
Bar graph demonstrating the peak intracavernous pressure in response to cavernous nerve stimulation (CNS; 2 V) in young (4–6 months) and aged (18–22 months) wild type (WT), heterozygous (Hemi) and transgenic sickle-cell (Sickle) mice. For all panels, data expressed as mean values ± SEM. *P < 0.05 vs. young WT; **P < 0.05 vs. young Hemi; ρ = P < 0.05 vs. young Sickle; θ = P < 0.05 vs. young WT and young Hemi; ω = P < 0.05 vs. young WT and young Hemi.
There were no visually obvious morphologic abnormalities in the aged sickle-cell mice penes, such as megalophallic changes, gross necrosis, or changes in gross penile structure. However, no histological microscopic analyses were obtained.
Discussion
The results of the present study show that transgenic Sickle mice penes have severe end organ damage (increased collagen deposition and endothelial destruction) consistent with morphometric changes that occur in penes of men with ischemic priapism. The present study also demonstrates that Sickle mice have increased resting ICP and significantly increased erectile responses to CNS. Moreover, we also demonstrate that Sickle mice have increased frequency of erections pre- and post-CNS. Importantly, these mice do develop ED as they age, suggesting that the priapism phenotype does abate with increasing age consistent with the human sickle-cell phenotype.
Priapism is associated diverse disease states, and a number of clinical contexts have risk associations for developing the disorder. Etiological categories include trauma, neurological conditions (multiple sclerosis, spinal cord tumor with compression), hematologic dyscrasias (sickle-cell disease, thrombophilia, and thalassemia), malignancies, intracavernous injection therapy (prostaglandin E1, papaverine, and combination therapy), as well as idiopathic circumstances [1,2]. Low-flow (ischemic) priapism is more prevalent than high-flow (nonischemic) priapism [1]. The former is painful, and blood–gas analysis reveals hypoxia. Ischemic priapism is characterized by hypoxia, hypercapnia, and acidosis [22]. The overall incidence of priapism ranges from 0.34 to 1.5 cases per 100,000 person-years [23,24]. The estimation of incidence rates of priapism also depends on the population of patients under study.
One category of patients with a high prevalence of recurrent priapism is men with sickle-cell disease. The lifetime probability for the development of clinically significant priapism is as high as 42% in men with sickle-cell disease [10]. A higher prevalence of ED is found in sickle-cell patients that suffer with recurrent priapism [10,25]. A recent investigation reported that African-American men with sickle-cell disease and ischemic priapism give a history of prior events; however, few ever receive education regarding the emergent nature of the condition, and thus present in a delayed fashion [26]. The precise mechanism of priapism in patients with sickle-cell disease remains elusive. The assumed mechanism is that normal erection decreases the oxygen tension in the corpora cavernosa, predisposing to erythrocyte sickling, which leads to penile closed compartment syndrome. However, the precise molecular mechanisms that precondition the sickle-cell penile vasculature to respond greater to any degree of an erectogenic stimulus are unclear. Therefore, the development and characterization of a transgenic sickle-cell disease mouse model is imperative and the focus of this study. In this study, we show that young adult sickle-cell mice have episodes of enhanced erectile responses to a neurogenic-stimulus with increased frequency of erectile episodes before and after CNS. These in vivo erectile responses are consistent with a priapism phenotype. Importantly, they have an end organ change that are consistent with that of sickle-cell disease patients with recurrent ischemic priapism, and that is associated with increased fibrosis of the penile vascular bed. An important observation in this study is the development of ED in sickle-cell mice as they age, which is also consistent with the natural evolution of sickle-cell patients with recurrent ischemic priapism.
Chronic anoxia and ischemia of priapism results in severe smooth muscle damage, widespread necrosis, as well as destruction of the endothelial lining as determined by electron microscopy in men suffering from ischemic priapism [21]. In the present study, we found similar electron microscopic findings in Sickle mice penes. Sickle mice penes had increased smooth muscle hypertrophy and increased basement membrane in the corporal sinusoids consistent with fibrosis. They also had endothelial cell lining disruption and adherence of RBC to the sinusoids of the corpora cavernosa. An additional finding was the significantly increased vacuolization of the cells in Sickle mice penes. These data may be interpreted as progressive dysfunction of the endothelium as a result of cellular stress. Since we did not positively identify these vesicular structures with caveolar protein markers, it remains unclear whether the increase in vacuoles within the endothelium are due to structurally altered and dysfunctional caveolae. Caveolae structural changes, both decreased and increased caveolae number and augmented protein expression, are associated with systemic endothelial dysfunction and ED [18,27–30]. The mechanism by which this occurs is unclear, but likely involves the influence of sickle-cell disease and chronic hemolysis on structural integrity and regulation of key factors, which regulate caveolin function in the endothelium. Potential mechanisms may involve reactive oxygen species (ROS) generation, decreased endothelial nitric oxide synthase (eNOS) expression and function, and/or shear stress.
The true pathophysiology of sickle-cell disease-associated priapism is not completely understood. An early report by Beuzard made an observation that transgenic sickle-cell mice had priapism [13]. Additionally, Mi and colleagues showed in vitro enhanced corporal smooth muscle relaxation to electrical field stimulation in isolated corporal strips from Sickle mice [9]. However, the methods used to make these observations were not critically performed in a standard in vivo erection physiological setup, and thus the conclusion that these mice had priapism was unsupported. The goal of this study was to characterize the morphological changes and in vivo erectile responses to CNS in Sickle mice and compare these with cellular and physiological indices of WT and Hemi mice. We have shown in this study for the first time that transgenic Sickle mice indeed have a priapism phenotype in vivo, with changes occurring in the penile vasculature that are similar to end organ changes that occur in men with sickle-cell disease. The ultimate aim of our laboratory is to study the pathogenesis of this disorder, as it relates to corporal smooth muscle biology and the biochemical regulation of erectile responses in priapism in vivo. The establishment of a transgenic sickle-cell disease mouse model will allow us and others to study the in vivo erectile responses and molecular analyses involved in the development of sickle-cell disease associated-priapism in an intact animal.
There are limitations to this transgenic sickle-cell animal model that should be acknowledged. Sickle mice demonstrate a wide spectrum of hematologic and histopathologic findings that are similar to those found in the human phenotype, but there are some differences [31]. Similarities include erythrocytic sickling, vascular ectasia, intravascular hemolysis, cardiomegaly, glomerulo-sclerosis, multiorgan infarcts, and hemorrhages to name a few. Important differences from humans is significant splenomegaly, more severe hepatic infarcts, and less severe pulmonary manifestiations. In the present study, we have demonstrated that penile end organ effects of priapism in the Sickle mice are similar to the effects that occur in the penis of men with sickle-cell disease.
We have recently proposed that priapism is a result of defective endothelial NO signaling with phosphodiesterase type 5 (PDE5) dysregulation in the penis [2,4,5,32–34]. PDE5 is a crucial molecular regulator of the erectile response and influences the overall vascular tone in the penis. We demonstrated that eNOS–/– mutant mice have an exaggerated erectile response to CNS and have phenotypic changes in erectile function consistent with priapism [4,5]. In this initial observation, we demonstrated that recurrent priapism is a manifestation of defective PDE5 regulatory function in the penis, resulting from altered endothelial NO/cyclic guanosine monophosphate (cGMP) signaling in the organ [4,5]. Molecular alterations in the NO/cGMP signaling cascade leads to a more relaxed penile vascular bed via downregulation of PDE5, so that any given amount of erectogenic stimulus or nocturnal erections will result in enhanced corporal smooth muscle relaxation and priapism. Another potential cause of enhanced corporal smooth muscle relaxation in sickle-cell disease-associated priapism is elevated penile adenosine levels [9]. The inducible isoform of heme oxygenase-1, which regulates vascular smooth muscle tone and responds to hypoxia, may also be involved in ischemic priapism [35]. Future studies will address potential pathophysiological mechanisms of priapism in transgenic sickle-cell disease mice. Additionally, the results of this study and future studies defining a role for “NO imbalance” and PDE5 dysregulation will likely add to the understanding of other vascular abnormalities that are manifested in sickle-cell disease and other hereditary and acquired hemolytic conditions [36,37].
Conclusion
Transgenic sickle-cell disease mice penes have severe end organ damage, namely increased fibrosis and endothelial cell disruption consistent with changes that occur in the penis of men with ischemic priapism. Additionally, they have exaggerated erectile responses in vivo consistent with a priapism phenotype. Therefore, the in vitro and in vivo results support the use of this animal model to study the pathophysiological mechanisms involved in priapism of sickle-cell disease.
Acknowledgments
The authors would like to thank Dr. Janice Taube, Warren Mason, and Carol Cooke for their help with analysis of trichrome stain and electron microscopy of the mice penes. Dr. Bivalacqua is supported by an American Urological Association (AUA) Foundation Astellas USA Foundation MD/PhD Fellowship and recipient of a National Kidney Foundation of Maryland Research Grant. Dr. Champion is supported in part by the Bernard A. and Rebecca S. Bernard Foundation, a Scientist Development Grant from the American Heart Association, the WW Smith Foundation, and NIH P50 Hl084946. He is a Recipient of the Zipes Distinguished Young Investigator Award of the American College of Cardiology, the Shin Chun-Wang Young Investigator Award and the Giles F. Filley Memorial Award from the American Physiological Society. Dr. Burnett is supported in part from NIH RO1 DK067223 and U54HL090515. Dr. Hsu and Gladwin are supported in part by federal funds from National Cancer Institute, NIH, under contract NO1-CO-12400 and the Intramural Program of the Vascular Medicine Branch of the National Heart, Lung, and Blood Institute of the NIH. The content of the article does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government.
Footnotes
Conflict of Interest: None.
Statement of Authorship
-
Conception and DesignTrinity J. Bivalacqua; Biljana Musicki; Arthur L. Burnett; Hunter C. Champion
-
Acquisition of DataTrinity J. Bivalacqua; Biljana Musicki; Hunter C. Champion
-
Analysis and Interpretation of DataTrinity J. Bivalacqua; Biljana Musicki; Lewis L. Hsu; Mark T. Gladwin; Arthur L. Burnett; Hunter C. Champion
-
Drafting the ArticleTrinity J. Bivalacqua; Arthur L. Burnett; Hunter C. Champion
-
Revising It for Intellectual ContentTrinity J. Bivalacqua; Biljana Musicki; Lewis L. Hsu; Mark T. Gladwin; Arthur L. Burnett; Hunter C. Champion
-
Final Approval of the Completed ArticleTrinity J. Bivalacqua; Biljana Musicki; Lewis L. Hsu; Mark T. Gladwin; Arthur L. Burnett; Hunter C. Champion
References
- 1.Montague DK, Jarow J, Broderick GA, Dmochowski RR, Heaton JP, Lue TF, Nehra A, Sharlip ID. Members of the Erectile Dysfunction Guideline Update Panel, Americal Urological Association. American Urological Association Guideline on the management of priapism. J Urol. 2003;170:1318–24. doi: 10.1097/01.ju.0000087608.07371.ca. [DOI] [PubMed] [Google Scholar]
- 2.Bivalacqua TJ, Burnett AL. Priapism: New concepts in the pathophysiology and new treatment strategies. Curr Urol Rep. 2006;7:497–502. doi: 10.1007/s11934-006-0061-6. [DOI] [PubMed] [Google Scholar]
- 3.Chinegwundoh F, Anie KA. Treatments for priapism in boys and men with sickle cell disease. Cochrane Database Syst Rev. 2004;4:CD004198. doi: 10.1002/14651858.CD004198.pub2. [DOI] [PubMed] [Google Scholar]
- 4.Champion HC, Bivalacqua TJ, Takimoto E, Kass DA, Burnett AL. Phosphodiesterase-5A dysregulation in penile erectile tissue is a mechanism of priapism. Proc Natl Acad Sci USA. 2005;102:1661–6. doi: 10.1073/pnas.0407183102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bivalacqua TJ, Liu T, Musicki B, Champion HC, Burnett AL. Endothelial nitric oxide synthase keeps erection regulatory function balance in the penis. Eur Urol. 2007;51:1732–40. doi: 10.1016/j.eururo.2006.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evliyaoglu Y, Kayrin L, Kaya B. Effect of pentoxifylline on veno-occlusive priapism-induced corporeal tissue lipid peroxidation in a rat model. Urol Res. 1997;25:143–7. doi: 10.1007/BF01037931. [DOI] [PubMed] [Google Scholar]
- 7.Munarriz R, Park K, Huang YH, Saenz de Tejada I, Moreland RB, Goldstein I, Traish AM. Reperfusion of ishcemic corporal tissue: Physiologic and biochemical changes in an animal model of ischemic priapism. Urology. 2003;62:760–4. doi: 10.1016/s0090-4295(03)00484-9. [DOI] [PubMed] [Google Scholar]
- 8.Ul-Hasan M, El-Sakka AI, Lee C, Yen TS, Dahiya R, Lue TF. Expression of TGF-beta-1 mRNA and ultrastructural alterations in pharmacologically induced prolonged penile erection in a canine model. J Urol. 1998;160:2263–6. doi: 10.1097/00005392-199812010-00097. [DOI] [PubMed] [Google Scholar]
- 9.Mi T, Abbasi S, Zhang H, Uray K, Chunn JL, Xia LW, Molina JG, Weisbrodt NW, Kellems RE, Blackburn MR, Xia Y. Excess adenosine in murine penile erectile tissues contributes to priapism via A2B adenosine receptor signaling. J Clin Invest. 2008;118:1491–501. doi: 10.1172/JCI33467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adeyoju AB, Olujohungbe AB, Morris J, Yardumian A, Bareford D, Akenova A, Akinyanju O, Cinkotai K, O’Reilly PH. Priapism in sickle cell disease; incidence, risk factors and complications—An international multicentre study. BJU Int. 2002;90:898–902. doi: 10.1046/j.1464-410x.2002.03022.x. [DOI] [PubMed] [Google Scholar]
- 11.Emond AM, Holman R, Hayes RJ, Serjeant GR. Priapism and impotence in homozygous sickle cell disease. Arch Intern Med. 1980;140:1434–7. [PubMed] [Google Scholar]
- 12.Nelson JH, Winter CC. Priapism: Evolution of management in 48 patients in a 22-year series. J Urol. 1977;117:455–8. doi: 10.1016/s0022-5347(17)58497-9. [DOI] [PubMed] [Google Scholar]
- 13.Beuzard Y. Transgenic mouse models of sickle cell disease. Curr Opin Hematol. 1996;3:150–5. doi: 10.1097/00062752-199603020-00008. [DOI] [PubMed] [Google Scholar]
- 14.Hsu LL, Champion HC, Campbell-Lee SA, Bivalacqua TJ, Manci EA, Diwan BA, Schimel DM, Cochard AE, Wang X, Schechter AN, Noguchi CT, Gladwin MT. Hemolysis in sickle cell mice causes pulmonary hypertension due to global impairment in nitric oxide bioavailability. Blood. 2007;109:3088–98. doi: 10.1182/blood-2006-08-039438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pászty C, Brion CM, Manci E, Witkowska HE, Stevens ME, Mohandas N, Rubin EM. Transgenic knockout mice with exclusively human sickle hemoglobin and sickle cell disease. Science. 1997;278:876–8. doi: 10.1126/science.278.5339.876. [DOI] [PubMed] [Google Scholar]
- 16.Bivalacqua TJ, Diner EK, Novak TE, Vohra Y, Sikka SC, Champion HC, Kadowitz PJ, Hellstrom WJ. A rat model of Peyronie’s disease associated with a decrease in erectile activity and an increase in inducible nitric oxide synthase protein expression. J Urol. 2000;163:1992–8. [PubMed] [Google Scholar]
- 17.Tokuyasu KT. A technique for ultracryotomy of cell susupensions and tissues. J Cell Biol. 1973;57:551–65. doi: 10.1083/jcb.57.2.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peterson TE, Poppa V, Ueba H, Wu A, Yan C, Berk BC. Opposing effects of reactive oxygen species and cholesterol on endothelial nitric oxide synthase and endothelial cell caveolae. Circ Res. 1999;85:29–37. doi: 10.1161/01.res.85.1.29. [DOI] [PubMed] [Google Scholar]
- 19.Hemnes AR, Zaiman A, Champion HC. PDE5A inhibition attenuates bleomycin-induced pulmonary fibrosis and pulmonary hypertension through inhibition of ROS generation and RhoA/Rho kinase activation. Am J Physiol Lung Cell Mol Physiol. 2008;294:L24–33. doi: 10.1152/ajplung.00245.2007. [DOI] [PubMed] [Google Scholar]
- 20.Bivalacqua TJ, Burnett AL, Hellstrom WJ, Champion HC. Overexpression of arginase in the aged mouse penis impairs erectile function and decreases eNOS activity: Influence of in vivo gene therapy of anti-arginase. Am J Physiol Heart Circ Physiol. 2007;292:H1340–51. doi: 10.1152/ajpheart.00121.2005. [DOI] [PubMed] [Google Scholar]
- 21.Spycher MA, Hauri D. The ultrastructure of the erectile tissue in priapism. J Urol. 1986;135:142–7. doi: 10.1016/s0022-5347(17)45549-2. [DOI] [PubMed] [Google Scholar]
- 22.Broderick GA, Gordon D, Hypolite J, Levin RM. Anoxia and corporal smooth muscle dysfunction: Mechanism for ischemic priapism. J Urol. 1994;151:259–62. doi: 10.1016/s0022-5347(17)34928-5. [DOI] [PubMed] [Google Scholar]
- 23.Eland IA, Van Der Lei J, Stricker BHC, Sturken-boom MJCM. Incidence of priapism in the general population. Urology. 2001;57:970–2. doi: 10.1016/s0090-4295(01)00941-4. [DOI] [PubMed] [Google Scholar]
- 24.Earle CM, Stuckey BGA, Ching HL, Wisniewski ZS. The incidence and management of priapism in western Australia: A 16 year audit. Int J Impot Res. 2003;15:272–6. doi: 10.1038/sj.ijir.3901018. [DOI] [PubMed] [Google Scholar]
- 25.Emond AM, Holman R, Hayes RJ, Serjeant GR. Priapism and impotence in homozygous sickle cell disease. Arch Intern Med. 1980;140:1434–7. [PubMed] [Google Scholar]
- 26.Bennett N, Mulhall J. Sickle cell disease status and outcomes of African-American men presenting with priapism. J Sex Med. 2008;5:1244–50. doi: 10.1111/j.1743-6109.2008.00770.x. [DOI] [PubMed] [Google Scholar]
- 27.Linder AE, Leite R, Lauria K, Mills TM, Webb RC. Penile erection requires association of soluble guanylyl cyclase with endothelial caveolin-1 in rat corpus cavernosum. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1302–8. doi: 10.1152/ajpregu.00601.2005. [DOI] [PubMed] [Google Scholar]
- 28.Bakircioglu ME, Sievert KD, Nunes L, Lau A, Lin CS, Lue TF. Decreased trabecular smooth muscle and caveolin-1 expression in the penile tissue of aged rats. J Urol. 2001;166:734–8. [PubMed] [Google Scholar]
- 29.Peterson TE, Poppa V, Ueba H, Wu A, Yan C, Berk BC. Opposing effects of reactive oxygen species and cholesterol on endothelial nitric oxide synthase and endothelial cell caveolae. Circ Res. 1999;85:29–37. doi: 10.1161/01.res.85.1.29. [DOI] [PubMed] [Google Scholar]
- 30.Darblade B, Caillaud D, Poirot M, Fouque M, Thiers JC, Rami J, Bayard F, Arnal JF. Alteration of plasmalemmal caveolae mimics endothelial dysfunction observed in atheromatous rabbit aorta. Cardiovasc Res. 2001;50:566–76. doi: 10.1016/s0008-6363(01)00251-6. [DOI] [PubMed] [Google Scholar]
- 31.Manci EA, Hillery CA, Bodian CA, Zhang ZG, Lutty GA, Coller BS. Pathology of Berkeley sickle cell mice: Similarities and differences with human sickle cell disease. Blood. 2006;107:1651–8. doi: 10.1182/blood-2005-07-2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burnett AL, Bivalacqua TJ, Champion HC, Musicki B. Feasibility of the use of phosphodiesterase type 5 inhibitors in a pharmacologic prevention program for recurrent priapism. J Sex Med. 2006;3:1077–84. doi: 10.1111/j.1743-6109.2006.00333.x. [DOI] [PubMed] [Google Scholar]
- 33.Burnett AL, Bivalacqua TJ. Glucose-6-phosphate dehydrogenase deficiency: An etiology for idiopathic priapism? J Sex Med. 2008;5:237–40. doi: 10.1111/j.1743-6109.2007.00631.x. [DOI] [PubMed] [Google Scholar]
- 34.Burnett AL, Bivalacqua TJ, Champion HC, Musicki B. Long-term oral phosphodiesterase 5 inhibitor therapy alleviates recurrent priapism. Urology. 2006;67:1043–8. doi: 10.1016/j.urology.2005.11.045. [DOI] [PubMed] [Google Scholar]
- 35.Jin YC, Gam SC, Jung JH, Hyun JS, Chang KC, Hyun JS. Expression and activity of heme oxygenase-1 in artificially induced low-flow priapism in rat penile tissues. J Sex Med. 2008;5:1876–82. doi: 10.1111/j.1743-6109.2008.00886.x. [DOI] [PubMed] [Google Scholar]
- 36.Kato GJ, Gladwin MT. Evolution of novel small-molecule therapeutics targeting sickle cell vasculopathy. JAMA. 2008;300:2638–46. doi: 10.1001/jama.2008.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wood KC, Hsu LL, Gladwin MT. Sickle cell disease vasculopathy: A state of nitric oxide resistance. Free Radic Biol Med. 2008;44:1506–28. doi: 10.1016/j.freeradbiomed.2008.01.008. [DOI] [PubMed] [Google Scholar]