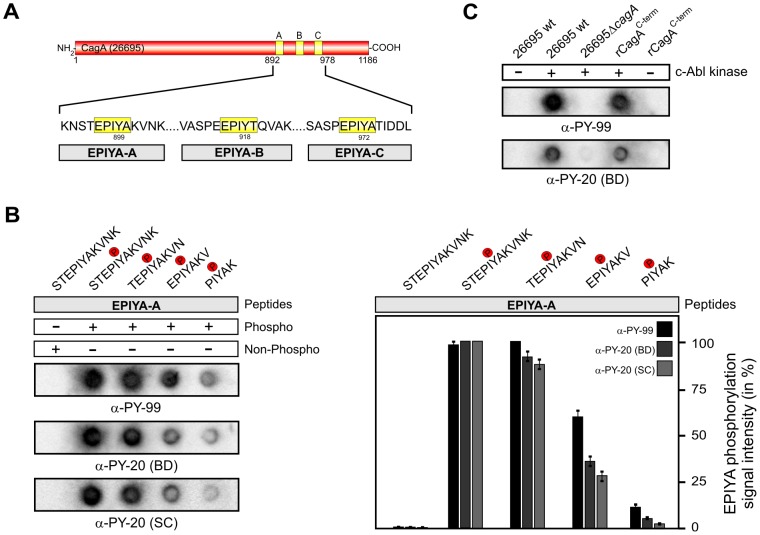
Figure 1. Short EPIYA-phosphopeptides of H. pylori CagA are sufficient for detection by α-phosphotyrosine antibodies.
(A) Typical Western CagA proteins of H. pylori such as that of strain 26695 [76] contain the EPIYA-A, EPIYA-B, and EPIYA-C segments as indicated. These motifs represent tyrosine phosphorylation sites, which can be phosphorylated by c-Abl and c-Src host kinases. (B) The indicated phospho- and non-phospho peptides of the EPIYA-A motif were synthesized and immobilized on PVDF membranes using a Dotblot apparatus. All Dotblots were probed with the indicated commercial phosphotyrosine antibodies and exposed as described in the Material & Methods section. Quantified spot intensities of the Dotblots from three independent experiments are shown to the right. Signal intensities were measured densitometrically with the Lumi-Imager F1 and revealed the percentage of phosphorylation signal per sample. The strongest spot on every Dotblot was set at 100% for each of the different α-phosphotyrosine antibodies as indicated. The results show that 11-mer and 9-mer phosphopeptides are sufficient for strong recognition by the antibodies. (C) Control Dotblot analyses used products of in vitro kinase reactions of c-Abl with either bacterial lysates (from H. pylori wild-type strain 26695 and isogenic ΔcagA mutant) or a purified recombinant CagA C-terminal fragment. Phosphorylated CagA proteins can be also detected by this Dotblot method using seven phosphotyrosine antibodies (Table 1), while the non-phosphorylated CagA forms cannot.