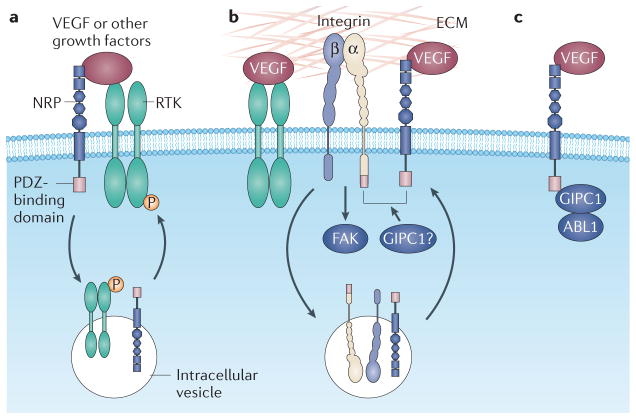
Figure 2. Receptor interactions that promote VEGF signalling in tumour cells, and the central role of NRPs.
a | Neuropilins (NRPs) interact with and potentiate the signalling function of growth factor receptor tyrosine kinases (RTKs), including vascular endothelial growth factor (VEGF) RTKs. This mode of regulation may be associated with internalization of the RTK and signalling from an intracellular compartment. Several growth factors, including hepatocyte growth factor, basic fibroblast growth factor, platelet-derived growth factor and transforming growth factor-β, directly interact with NRPs, but whether this binding is sufficient by itself to induce a signalling response is not known. b | NRPs also interact with specific integrins and activate their ability to bind to extracellular matrix (ECM) ligands, which results in the stimulation of integrin-mediated signalling through focal adhesion kinase (FAK). The RTK VEGF receptor 2 (VEGFR2) can also function in a similar capacity. In addition, NRPs may regulate integrin function by promoting their endocytic recycling. Both NRPs and specific integrin α-subunits (α5 and α6) contain a PDZ (PSD95, DLG and ZO1)-binding domain (Ser-Glu-Ala) at their carboxyl terminus, and PDZ proteins, such as the neuropilin-interacting protein GIPC1 might promote the association of these two classes of receptors. c | NRPs may also signal independently of other receptors, possibly by using their PDZ-binding domains to associate with signalling molecules such as ABL1. Note that these proposed mechanisms are not mutually exclusive, and there is the possibility that VEGF signalling in tumour cells involves the formation of macromolecular complexes that integrate components of these mechanisms.