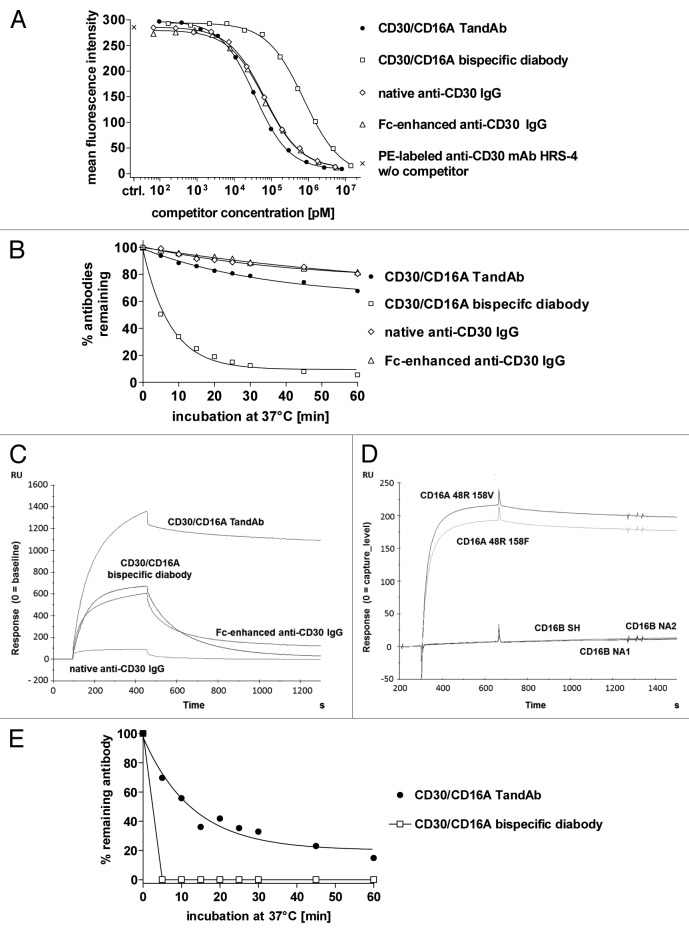
Figure 2. Binding of the TandAb, the diabody and IgGs to CD30 and CD16A. (A) Competition assay. 1x106 KARPAS-299 cells were incubated with 3 µg/mL (~20 nM) PE-conjugated anti-CD30 HRS-4 IgG together with the indicated concentrations of recombinant bispecific antibodies or anti-CD30 IgGs for 45 min at 37 °C. After washing, the fluorescence of 1 × 104 cells was measured with a flow cytometer, corrected for background staining with the corresponding isotype control and analyzed by nonlinear regression for calculation of IC50 and KD values. One out of two experiments is depicted. (B) Cell surface retention at 37 °C. Aliquots of KARPAS-299 cells were incubated with biotinylated CD30/CD16A TandAb and diabody or anti-CD30 IgG for 45 min on ice. After removing excess antibodies by washing, cells were incubated at 37 °C for the indicated periods of time. After repeated washing on ice, remaining antibodies were detected using Dylight488-conjugated streptavidin, and the fluorescence of 1 × 104 cells was analyzed by flow cytometry. The mean fluorescence values from time-point 0 were set to 100% and the percentage of remaining antibodies was determined using nonlinear regression analysis. Data shown are representative of two independent experiments. (C) The CD16A 48R158V Fc-fusion protein was covalently immobilized on a CM5 chip. The different antibodies (CD30/CD16A TandAb and diabody, and anti-CD30 IgGs,) were injected with a flow rate of 10 µL/min for 360 s and the dissociation time was set to 600 s. Background signals in the control flow cell (without immobilized antigen) were subtracted from signals in the test flow cell. Chips were regenerated with 10 mM Glycine-HCL, pH 2.0. (D) The CD16A allotypes, 48R158V and 48R158F, and the CD16B allotypes, SH, NA1 and NA2, were expressed in HEK-293 cells as Fc-fusion proteins and directly immobilized on chips. TandAb was injected with a flow rate of 10 µL/min for 360 s. Background signals in the control flow cell (without immobilized antigen) were subtracted from signals in the test flow cell. Dissociation time was set to 600 s. Chips were regenerated with 10 mM Glycine-HCL, pH 2.0. (E) Cell surface retention assay. 1 × 106 enriched human NK cells were stained with 1 µM of CD30/CD16A TandAb or diabody on ice, washed and incubated for indicated periods of time at 37 °C to allow dissociation. After washing, remaining antibodies were detected by anti-His IgG followed by FITC-conjugated goat anti-mouse IgG. The mean fluorescence values from time-point 0 were set to 100% and the percentage of remaining antibodies was plotted using nonlinear regression.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.