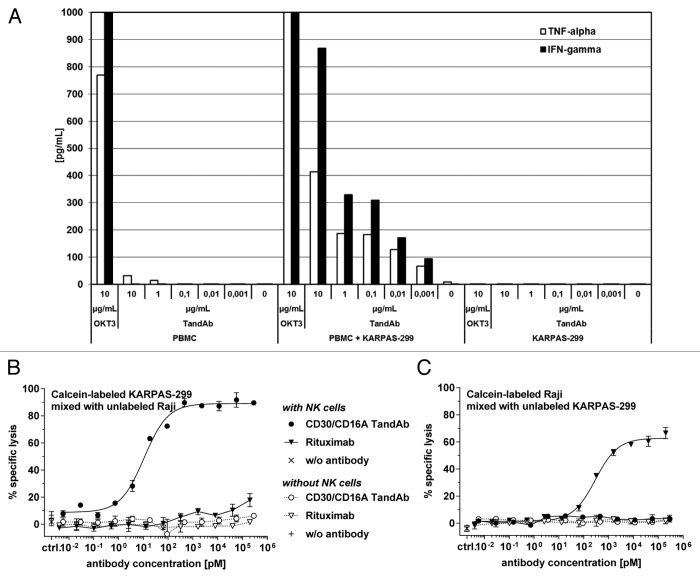
Figure 4. Specificity and safety in vitro. (A) Cytokine release in PBMC cultures in the presence of the CD30/CD16A TandAb. 5 × 105 human PBMC with and without 1 × 104 CD30+ KARPAS-299 cells, or 1 × 104 KARPAS-299 cells alone were cultured in the presence of increasing concentrations of the CD30/CD16A TandAb (denoted by TandAb; at 0.001–10 µg/mL), 10 µg/mL OKT3, or without antibody (denoted by 0). The concentrations of secreted TNF and IFN-γ were quantified using multiplexing after 24 h incubation. Results from one representative experiment are shown. (B and C) Assessment of bystander cell killing in a cytotoxicity assay with mixed target cells. 1 × 104 calcein-labeled CD20-/CD30+ KARPAS-299 target cells, combined with 1x104 unlabeled CD20+/CD30- Raji target cells (B) or 1 × 104 unlabeled KARPAS-299 combined with calcein-labeled Raji cells (C), were incubated for 3 h, together with increasing concentrations of the CD30/CD16A TandAb or rituximab (MabThera, Roche, Hertfordshire, UK), in the presence or absence of 5 × 104 enriched human NK cells as effectors for 3 h in a cytotoxicity assay. The percentage of specific target cell lysis was calculated and the mean and SD from duplicates were plotted.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.