Fig. 3.
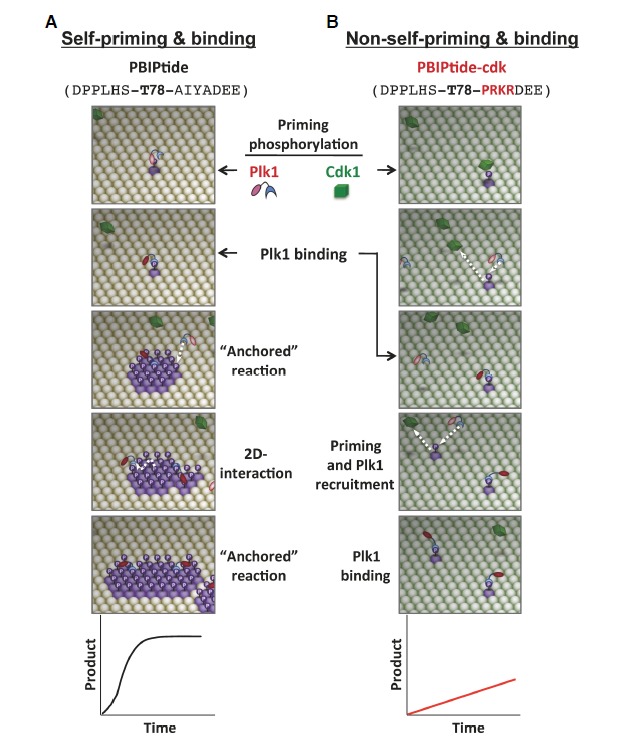
A comparative illustration of self-priming- versus non-self-priming-based biochemical processes. The schematic diagrams are based on the results obtained with a pair of ELISA-based kinase assays that utilize either Plk1-phosphorylatable PBIPtide (A) or Cdk1-phosphorylatable PBIPtide-cdk (B) as substrates (Park et al., 2011). The sequences for PBIPtide (A) and the PBIPtide-cdk mutant (B) are shown in parentheses with the mutations highlighted in red. The T78 residue is indicated in boldface type. Plk1 is initially at an inactive state (pink kinase domain), which becomes partially activated (red kinase domain) upon binding to a PBD-docking site. Phosphorylation of the T210 residue at the activation loop of Plk1 further activates the enzyme (Lee and Erikson, 1997; Xu et al., 2013). Straight arrows indicate the incoming or outgoing movement of a molecule, whereas the curved arrow in (A) indicates the lateral movement of a molecule on a two-dimensional surface. Graphs depict the reaction kinetics of each mechanism. See Supplementary Movie S1.
