Fig. 4.
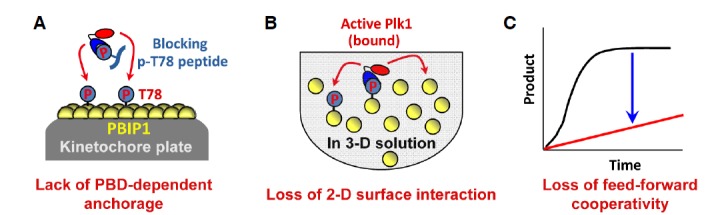
Loss of feed-forward cooperativity by either the interference of a PBD-dependent interaction or the abrogation of a two-dimentional surface interaction. (A) The provision of a PBD-binding p-T78 peptide prevents Plk1 from binding to the p-T78 motif of PBIP1 localized at the kinetochore plate. In the absence of PBD-dependent anchorage onto the p-T78 motif, Plk1 fails to efficiently phosphorylate PBIP1. (B) Under the conditions where two-dimensional surface interaction is abrogated, Plk1 is not able to take advantage of PBD-dependent anchored reactions onto neighboring substrates. Rather, it phosphorylates its substrates through a three-dimensional stochastic processes. (C) Loss of either one of the PBD-dependent anchorage and the two-dimensional surface interaction is sufficient to prevent Plk1 from phosphorylating its substrates in a cooperative manner.
