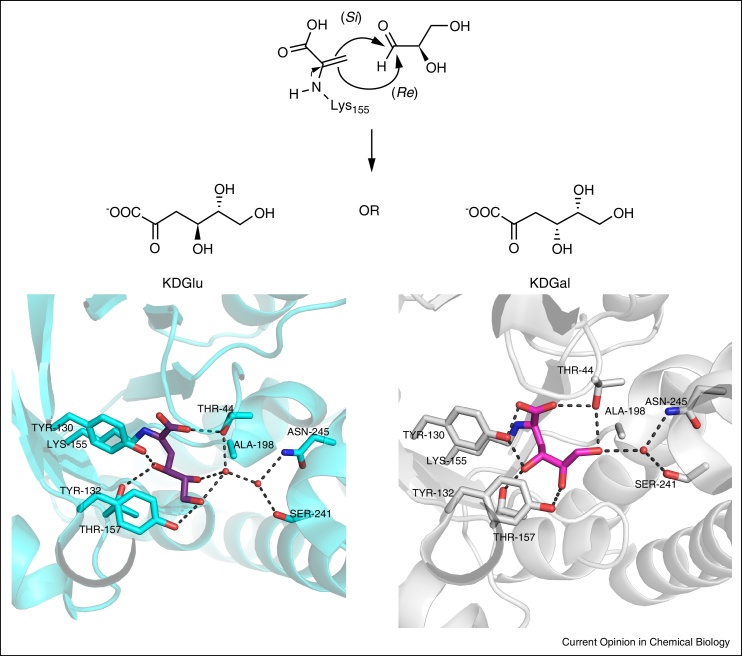
Figure 1.
The top panel shows the attack of the lysine bound enamine form of pyruvate onto the either the Si or Re face of d-glyceraldehyde, to produce either KDGlu or KDGal. The bottom panel shows the binding interactions of KDGlu (on the left) and KDGal (on the right) in the active site of KDGA. Differences in the stabilization of C5-OH and C6-OH of KDGlu and KDGal can be seen. The C5-OH residue of d-KDGal was shown to hydrogen bond directly with Tyr-132 whereas the C5-OH residue of d-KDGlu makes water mediated hydrogen-bonding interactions within the active site. There are also differences in the bonding of the terminal C6-OH residue; in d-KDGal it interacts directly with Thr-44 whereas in d-KDGlu it is directly stabilized by hydrogen bonding with Tyr-132 and water mediated hydrogen bonding.